Abstract
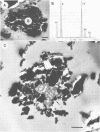
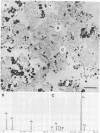
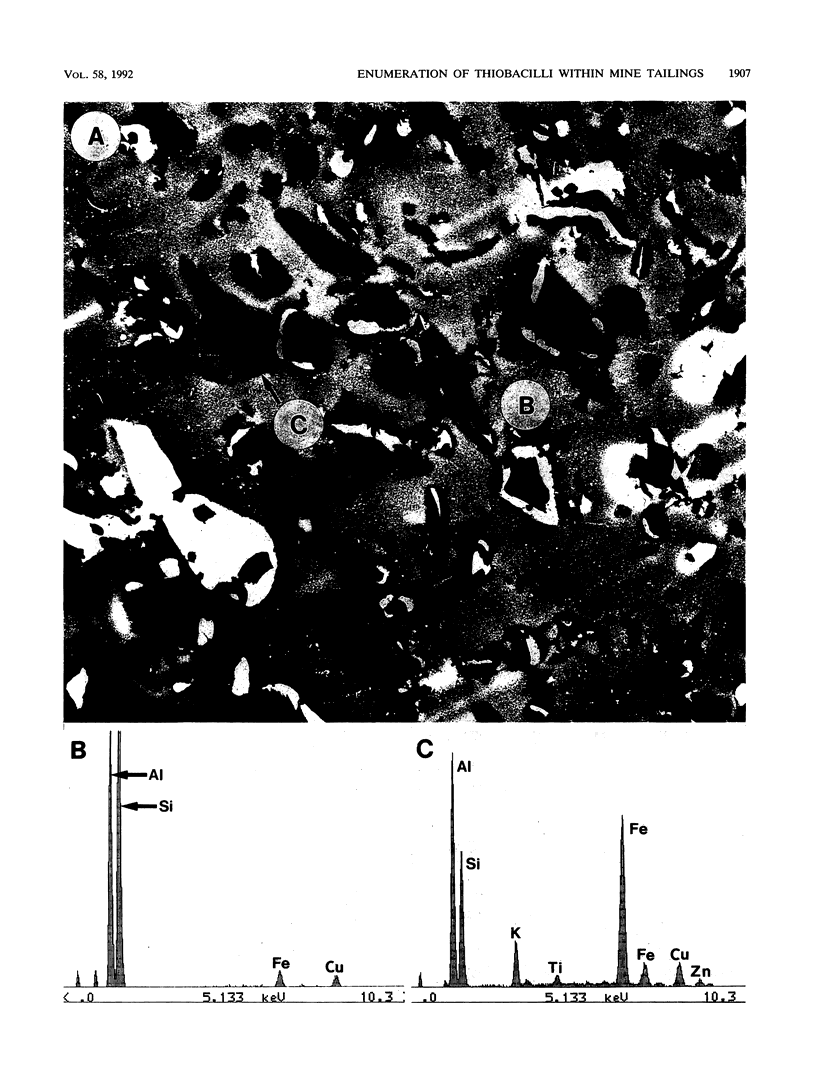
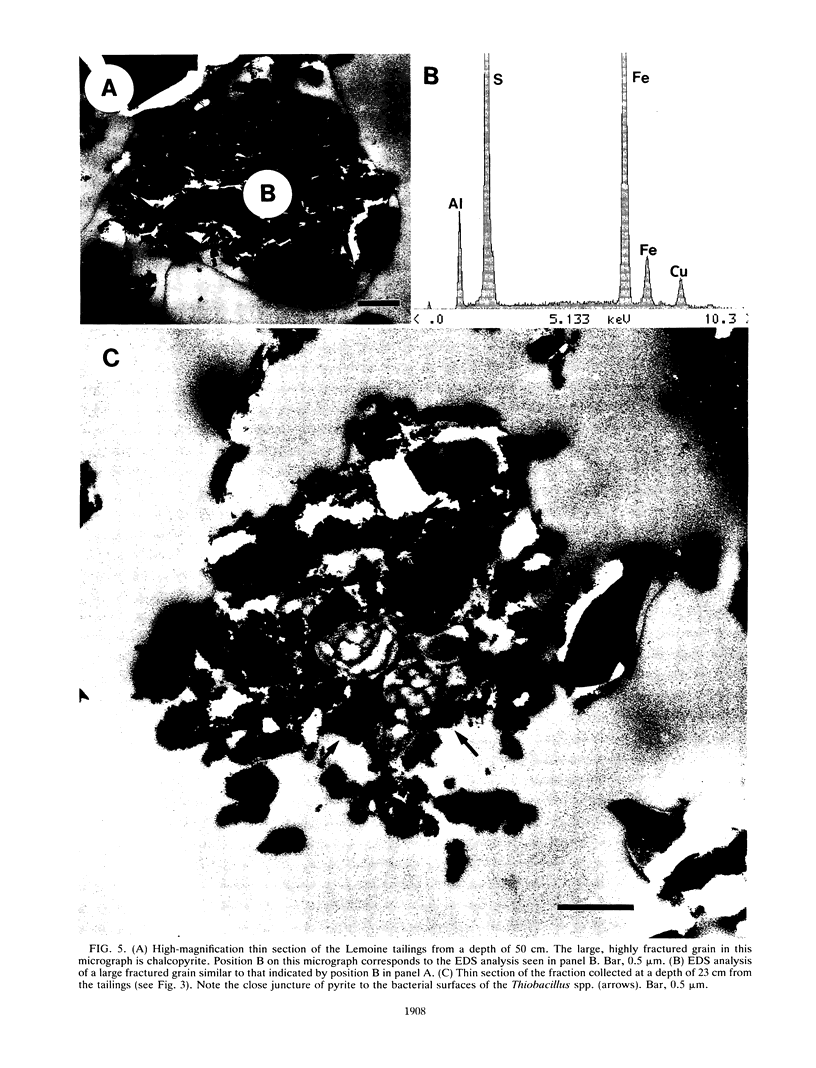
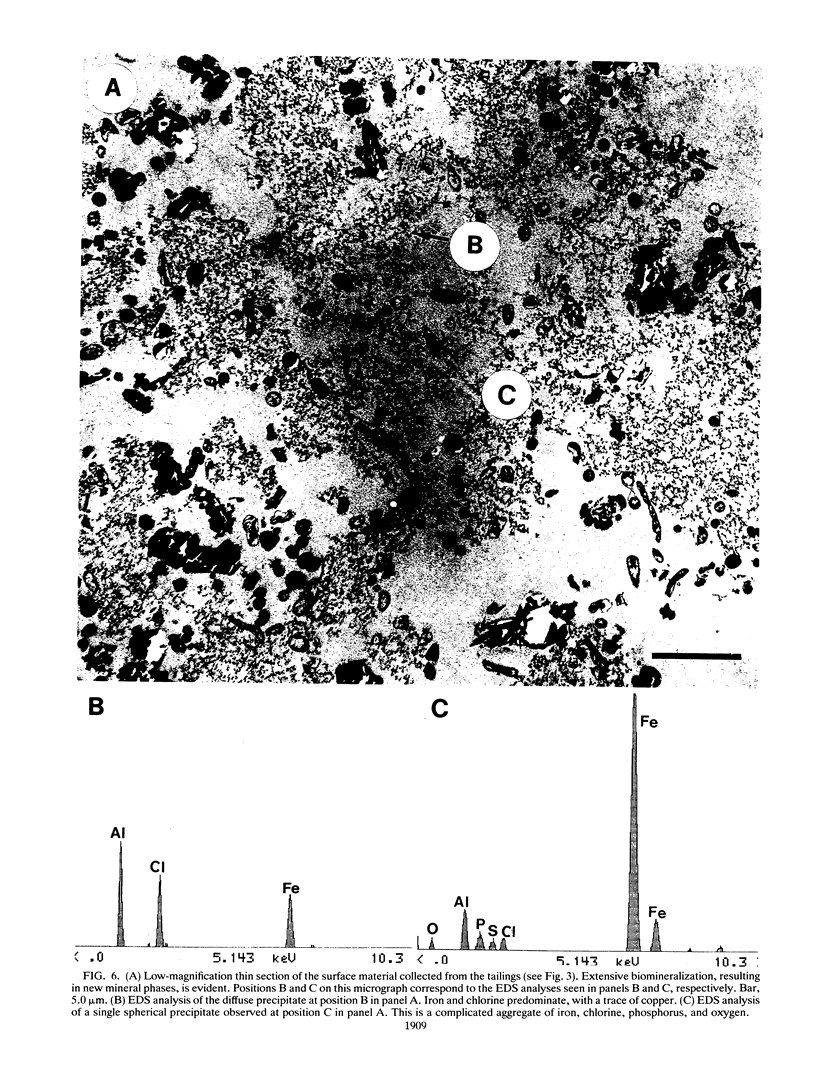
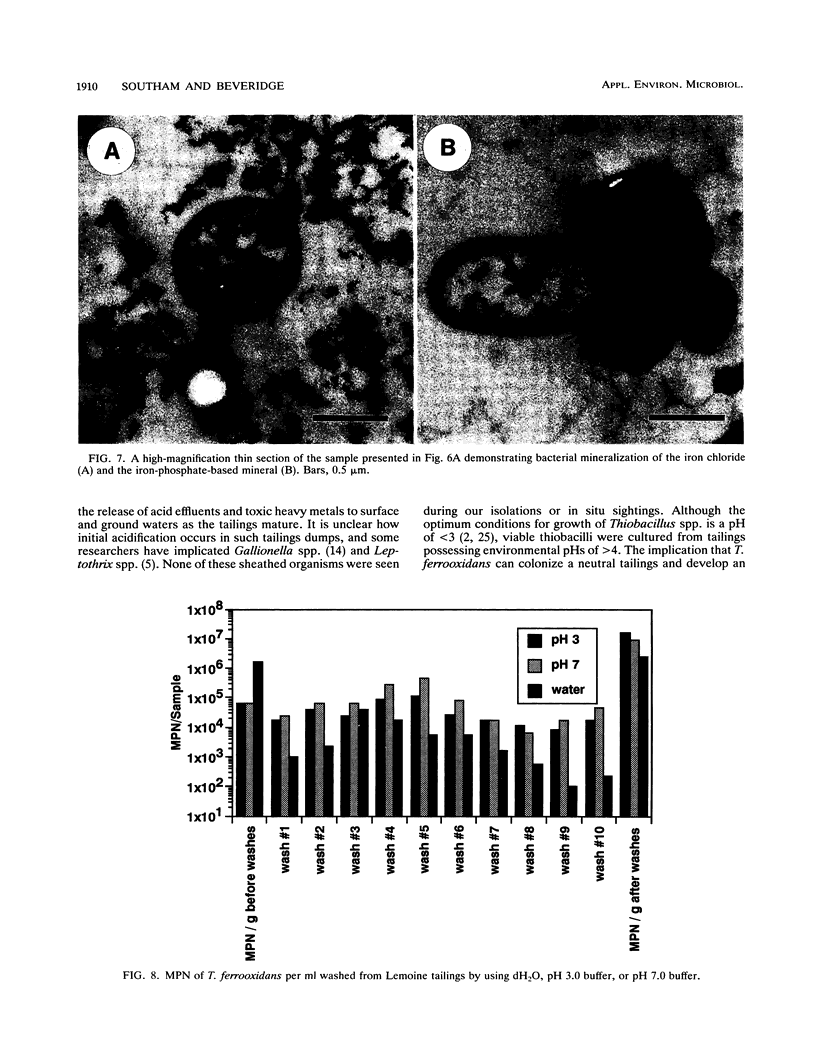
The Lemoine tailings of Chibougamau, Quebec, Canada, were deposited as a pH-neutral mineral conglomerate consisting of aluminum-silicates, iron-aluminum-silicates, pyrite, chalcopyrite, and sphalerite. These tailings are colonized by an active population of Thiobacillus ferrooxidans which is localized to an acid zone occupying 40% of the tailings' surface. This population peaked at 7 × 108 most probable number per gram of tailings during July and August 1990 and extended to a depth of 40 cm from the surface. Examination of samples over this depth profile by transmission electron microscopy and electron dispersive spectroscopy revealed a microbially mediated mineral transition from sulfides (below 40 cm) to chlorides and phosphates (at the surface). Silicate minerals were unaltered by microbial action. Transmission electron microscopy showed a tight association between Thiobacillus species and the sulfide minerals, which helps account for their prominence in tailings environments. Accurate enumeration of T. ferrooxidans from tailings required the disruption of their bonding to the mineral interface. Vortexing of a 10% aqueous suspension of the tailings material prior to most-probable-number analysis best facilitated this release. Even though heavy metals were highly mobile under acidic conditions at the Lemoine tailings, it was evident by transmission electron microscopy and electron dispersive spectroscopy that they were being immobilized as bona fide fine-grain minerals containing iron, copper, chlorine, phosphorus, and oxygen on bacterial surfaces and exopolymers. This biomineralization increased with increasing bacterial numbers and was most evident in the upper 3 cm of the acidic zone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonen L., Tuovinen O. H. Bacterial oxidation of sulfide minerals in column leaching experiments at suboptimal temperatures. Appl Environ Microbiol. 1992 Feb;58(2):600–606. doi: 10.1128/aem.58.2.600-606.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro A. M., Chamorro D., Seeger M., Arredondo R., Peirano I., Jerez C. A. Effect of external pH perturbations on in vivo protein synthesis by the acidophilic bacterium Thiobacillus ferrooxidans. J Bacteriol. 1991 Jan;173(2):910–915. doi: 10.1128/jb.173.2.910-915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRALEY S. A., Sr, KINSEL N. A., LEATHEN W. W. Ferrobacillus ferrooxidans: a chemosynthetic autotrophic Bacterium. J Bacteriol. 1956 Nov;72(5):700–704. doi: 10.1128/jb.72.5.700-704.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. C., Tributsch H. Bacterial leaching patterns on pyrite crystal surfaces. J Bacteriol. 1978 Apr;134(1):310–317. doi: 10.1128/jb.134.1.310-317.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemel W. N., Brock T. D. pH of very acid soils. Nature. 1971 Feb 19;229(5286):574–574. doi: 10.1038/229574a0. [DOI] [PubMed] [Google Scholar]
- Ferris F. G., Schultze S., Witten T. C., Fyfe W. S., Beveridge T. J. Metal Interactions with Microbial Biofilms in Acidic and Neutral pH Environments. Appl Environ Microbiol. 1989 May;55(5):1249–1257. doi: 10.1128/aem.55.5.1249-1257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Harrison A. P., Jr The acidophilic thiobacilli and other acidophilic bacteria that share their habitat. Annu Rev Microbiol. 1984;38:265–292. doi: 10.1146/annurev.mi.38.100184.001405. [DOI] [PubMed] [Google Scholar]
- JONES G. E., STARKEY R. L. Surface-active substances produced by Thiobacillus thiooxidans. J Bacteriol. 1961 Nov;82:788–789. doi: 10.1128/jb.82.5.788-789.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I., Takeuchi T. L., Yuthasastrakosol T. D., Oh J. K. Ferrous Iron and Sulfur Oxidation and Ferric Iron Reduction Activities of Thiobacillus ferrooxidans Are Affected by Growth on Ferrous Iron, Sulfur, or a Sulfide Ore. Appl Environ Microbiol. 1990 Jun;56(6):1620–1626. doi: 10.1128/aem.56.6.1620-1626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]