Abstract
According to the free radical theory of aging, reactive oxygen species cause oxidative damage, proposed to be an underlying factor of the aging process. In the current study, we have used electron paramagnetic resonance spin labeling, measurements of protein carbonyl content, an index of protein oxidation, and determination of the activity of glutamine synthetase (an oxidatively sensitive enzyme) to report that cortical synaptosomal membranes from the senescence accelerated-prone (SAMP8) mouse showed structural characteristics of free radical oxidative stress relative to the senescence accelerated-resistant (SAMR1) mouse. The SAMP8 mouse exhibited a decrease in the relevant EPR parameter consistent with oxidative stress (P < 0.002), a decreased glutamine synthetase activity (P < 0.05), and an increased protein carbonyl content (P < 0.01) compared with these parameters in the SAMR1 mouse. Further, because free radical trapping compounds have been demonstrated to extend maximum life span and improve cognition in SAMP8 mice, we investigated the protective nature of the known free radical scavenger, N-tert-butyl-α-phenylnitrone (PBN), on the physical state of cortical synaptosomal membrane proteins. For 14 days, SAMR1 and SAMP8 mice were injected with 30 mg/kg PBN while the controls were injected with the corresponding volume of saline. Characteristic of less oxidized systems, cortical synaptosomal membranes from the PBN-injected SAMP8 mouse exhibited a return toward normal values of the relevant EPR parameter [the MI = +1 low-field weakly immobilized line/MI = +1 low-field strongly immobilized line (W/S) ratio of a protein-specific spin label] (P < 0.001) compared with that from saline-injected SAMP8 mice. In SAMR1 mice, in contrast to SAMP8, there was no significant change in the conformation of membrane proteins or protein carbonyl content of cortical synaptosomal membranes from the PBN-injected and saline-injected SAMR1 mice, showing that PBN itself did not induce conformational changes in cortical synaptosomal membrane proteins. The results are discussed with reference to the use of free radical scavengers as potential anti-aging agents.
Keywords: aging, membrane proteins, anti-aging agents
Aging and age-related neurological disorders, especially Alzheimer disease and stroke, affect millions of people worldwide. Many hypotheses have been developed to explain aging and age-related neurodegenerative disorders. One of the most compelling, and one for which our laboratory has contributed extensively, is the role of free radical-induced oxidative stress in aging, stroke, Alzheimer disease, Parkinson disease, and Huntington disease (1–20). The free radical theory of normal aging proposes that the slow generation of oxygen free radicals, an unavoidable consequence of life in an aerobic environment, results in cumulative damage to critical cellular components, and eventually leads to age-related pathology (21). A consensus is emerging that free radical processes do play an important role in the etiology of many age-related disorders (10, 11, 16, 17, 22, 23), although the specific mechanisms for the free radical generation and consequent oxidative stress differ among the pathologies. Free radical-mediated damage to neuronal membrane components have been implicated in the etiology of many neurodegenerative disorders, especially Alzheimer disease (2, 8, 10, 11, 16, 17), in which one of the most dominant risk factors is age (24). In addition to neurodegenerative disorders, both progeria and Werner disease, conditions of accelerated human aging, have been shown to be associated with free radical oxidative stress (18, 20). Hence, it is imperative to learn more about free radical involvement in aging.
Established by Takeda et al. (25), the senescence accelerated mouse (SAM) is a novel murine model of accelerated aging. The SAMP8 strain (senescence accelerated-prone) exhibits a shortened life span and early signs of various indices of aging; in contrast, the SAMR1 strain (senescence accelerated-resistant), which is closely related genetically, does not (26). The indices of aging found in the SAMP8 mouse include a moderate to severe decline of activity, hair loss and lack of hair glossiness, skin coarseness, periopthalamic lesions, and increased lordokyphosis of the spine. In addition, the SAMP8 strain exhibits age-related deterioration in memory and learning (27, 28) and, therefore, is thought to be a good model for aging.
Consistent with other researchers (19, 20), our laboratory previously demonstrated that free radical oxidative stress led to increased amounts of oxidized proteins in cortical synaptosomal membranes (1, 3–12), a result also seen in aged gerbils (1). Therefore, if free radicals were involved in aging, we hypothesized that SAMP8 age-accelerated mice should show evidence of cortical membrane protein oxidation. To investigate this hypothesis, three indices of free radical oxidative stress were used: (i) the MI = +1 low-field weakly immobilized line/MI = +1 low-field strongly immobilized line (W/S) ratio of a protein-specific spin label, an EPR parameter reflective of protein–protein interactions (29) and known to be decreased in free radical oxidative stress (1, 3–12); (ii) the activity of glutamine synthetase (GS), an oxidatively sensitive enzyme (19); and (iii) protein carbonyl levels, shown by Stadtman and others to be increased in aging (19, 20, 30, 31). Our laboratory also previously demonstrated that prior in vivo injection of the brain-accessible free radical scavenger, N-tert-butyl-α-phenylnitrone (PBN) in animals exposed to oxidative stress via ischemia/reperfusion injury or hyperoxia ameliorated alterations in the physical state of cortical synaptosomal membrane proteins (4, 12). Therefore, we reasoned that injection of age-accelerated mice with PBN would modulate the elevated protein oxidation (4, 12), but not so in the SAMR1 age resistant-prone mice. We report here that brain membrane alterations characteristic of free radical damage were found in accelerated senescence in the SAMP8 mouse, and these alterations can be inhibited by prior injection with PBN.
MATERIALS AND METHODS
Chemicals.
Ultra-pure sucrose was obtained from Sigma. The protease inhibitors leupeptin, pepstatin A, and aprotinin were purchased from Calbiochem. Ultrapure PBN was obtained from Centaur Pharmaceuticals (Sunnyvale, CA). The protein-specific spin label 2,2,6,6,-tetramethyl-4-maleimidopiperidine-1-oxyl (MAL-6) was purchased from Sigma. All remaining chemicals were obtained from Sigma in the highest possible purity.
Animals.
All protocols were approved by the University of Kentucky Animal Care and Use Committee. The SAM mice were originally donated by Toshio Takeda (Kyoto University). The 10-month-old adult mice were housed in the University of Kentucky Medical Center Animal Care Facility. The mice received 12 hr light/12 hr dark conditions and free access to water and standard Rodent Laboratory Chow (Purina). For those animals who were to receive the free radical scavenger PBN, this agent, dissolved in physiological saline, was administered i.p. daily for 14 days at a dose of 30 mg/kg, a concentration that was protective against hyperoxia damage (12). The last dose of PBN occurred 24 hr before utilizing the brain as described below. Control animals received corresponding injections of physiologic saline.
Animals were killed by decapitation. The whole brain was removed and the neocortex was obtained. The neocortex was suspended in approximately 20 ml of isolation buffer (0.32 M sucrose/10 mM Hepes, pH 7.4/4 mg/ml leupeptin/4 mg/ml pepstatin/5 mg/ml aprotinin/20 mg/ml type II-S soybean trypsin inhibitor/0.2 mM phenylmethylsulfonyl fluoride/2 mM EDTA/2 mM EGTA). The individual cortices (not pooled) were homogenized in a Wheaton Scientific 30-ml motor-driven Potter-type homogenizer with a Teflon pestle as described (1, 4).
Synaptosome Preparation.
Synaptosomes were purified as described (1, 2). The crude homogenate was removed and respun at 20,000 × g at 4°C for 10 min. The resulting pellet was resuspended in the isolation buffer and layered on a discontinuous sucrose gradient (9 ml of 1.18 M sucrose, pH 8.5/9 ml of 1.0 M sucrose, pH 7.4/9 ml of 0.85 M sucrose, pH 7.4, each containing 2 mM EDTA/2 mM EGTA/10 mM Hepes). The samples were then spun at 82,500 × g at 4°C for 120 min in a Beckman swinging-bucket rotor. Synaptosomes were removed from the 1.18/1.0 M sucrose interface and resuspended in 20 ml of lysing buffer (10 mM Hepes/2 mM EDTA/2 mM EGTA, pH 7.4). The samples were then centrifuged at 32,000 × g at 4°C for 10 min. The pellet was removed and resuspended in lysing buffer and spun down two more times. After the third wash, the protein concentration was determined by the Lowry method (32).
Spin Labeling.
Spin labeling of synaptosomal membrane proteins was performed as described (3, 33). Lysed synaptosomal membranes were labeled with the protein-specific spin label MAL-6. After incubation for 16–18 hr at 4°C with 20 μg MAL-6/mg protein, samples were washed six times in lysing buffer to remove excess spin label. The pellet was then resuspended in approximately 400 ml lysing buffer and allowed to come to room temperature. EPR spectra were acquired on a Bruker model 300 EPR spectrometer (Bruker, Billerica, MA) operating at an incident microwave power of 18 mW, a modulation amplitude of 0.4 G, a time constant of 1.28 msec, and a conversion time of 10 msec.
Protein Carbonyl Levels.
Before ultracentrifugation, the synaptosomal cytosolic supernatant was removed and the protein concentration was determined by the Lowry method. Protein carbonyl levels of cortical synaptosomal cytosolic proteins were then determined as adducts of 2,4-dinitrophenylhydrazine according to the method of Oliver et al. (20).
GS Assay.
Before ultracentrifugation, the supernatant was removed and protein concentration was determined by the Lowry method (32). GS activity of cortical proteins was then determined according to the method of Rowe et al. (34) as modified by Miller et al. (35).
Data Analysis.
The data were analyzed by ANOVA and Student’s t tests. A value of P < 0.05 was considered statistically significant.
RESULTS
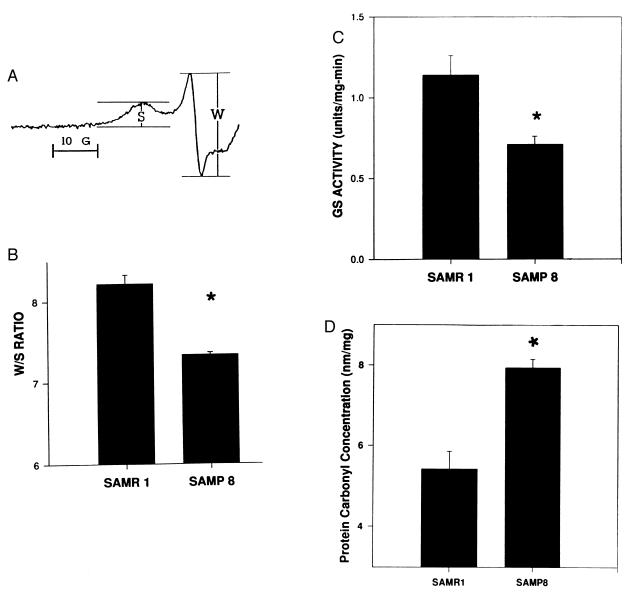
The relevant EPR parameter of MAL-6-labeled membrane proteins is the W/S ratio (Fig. 1), which has been extensively studied with respect to cytoskeletal perturbations in both erythrocyte and brain synaptosomal membranes (3–12, 29, 33, 36, 37). A decrease in the W/S ratio indicates increased steric hindrance of the spin label mobility as a consequence of altered conformation of or decreased segmental motion in spin labeled proteins and/or changes in the interactions among membrane proteins. Previous studies in our laboratory employing several oxidative conditions including Fenton chemistry to produce hydroxyl radicals (3), hyperoxia (1, 12), sepsis-related lipopolysaccharide (37), ischemia-reperfusion (4–7), β-amyloid-derived free radicals (8–11, 16, 17), and menadione (36) revealed that increased protein oxidation is associated with a decrease in the W/S ratio.
Figure 1.
(A) Typical MI = +1 low-field EPR line of MAL-6-labeled synaptosomal membrane proteins showing the W and S spectral components. (B) Data represent the average W/S ratio of MAL-6 covalently bound to proteins in cortical synaptosomal membranes from SAMR1 and SAMP8 mice. Error bars indicate SEM for nine and three animals, respectively. ∗, P < 0.002. (C) Data represent the average cortical GS activity of SAMR1 and SAMP8 mice. Error bars indicate SEM for nine and three animals, respectively. ∗, P < 0.05. (D) Data represent the average protein carbonyl content of cortical proteins from SAMR1 and SAMP8 mice. Error bars indicate SEM for six and three animals, respectively. ∗, P < 0.001.
Consistent with oxidative stress in age-accelerated mice, Fig. 1B shows that the W/S ratio is decreased significantly in cortical synaptosomal membrane proteins from SAMP8 mice compared with that from SAMR1 mice (P < 0.002). Likewise, Fig. 1C shows that the activity of the oxidatively sensitive enzyme GS from SAMP8 brain is significantly decreased compared with that from SAMR1 mouse brain (P < 0.05).
Stadtman and others showed that cytosolic protein carbonyl levels are increased in protein oxidation (19, 20, 30, 31, 38). To determine whether cytosolic protein carbonyl levels of cortical synaptosomal membranes were increased in the SAMP8 mouse relative to those from SAMR1, the 2,4-dinitrophenylhydrazine adducts were measured. The results suggest that the protein carbonyl levels of cytosolic proteins in SAMP8 cortical synaptosomes were significantly greater than those of SAMR1 (Fig. 1D; P < 0.001), which is consistent with the EPR results.
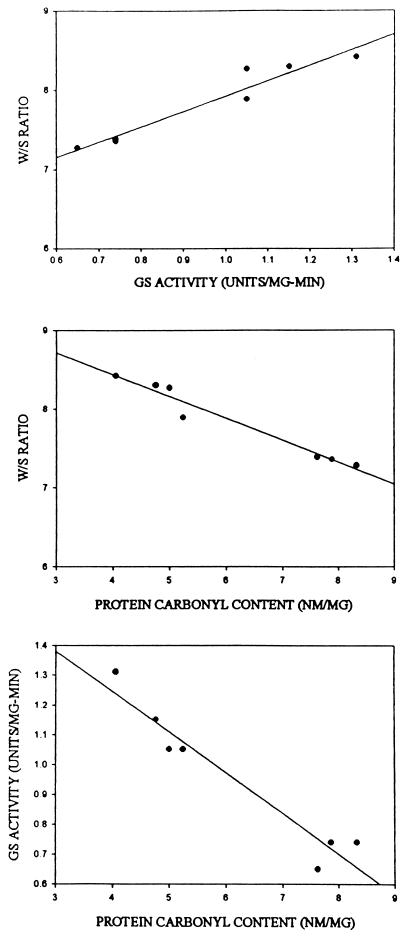
Regression analyses suggested that brain membrane structural alterations in the SAM mice, measured by the W/S ratio of MAL-6, apparently were correlated to both diminution of GS activity and increased carbonyl content (Fig. 2), which is consistent with the notion that these parameters may index the same molecular processes associated with accelerated aging.
Figure 2.
Regression analysis of SAM data (SAMR1 and SAMP8) correlating: W/S ratio and GS activity, r2 = 0.93 (Top); W/S ratio and protein carbonyl content, r2 = 0.96 (Middle); and GS activity and protein carbonyl content, r2 = 0.93 (Bottom).
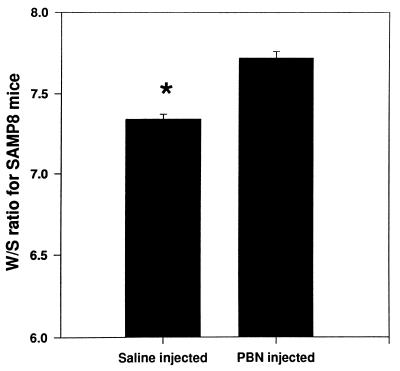
If in accelerated aging these conformational alterations in cortical synaptosomal membrane proteins, as assessed by protein-specific spin labeling, were due to free radical-associated effects, then the brain-accessible free radical scavenger PBN should modulate these alterations. The W/S ratio of MAL-6, covalently coupled to proteins in cortical synaptosomal membranes, is an oxidatively sensitive parameter whose value is lower in oxidative stress (1, 3–12, 36, 37). Fig. 3 suggests that the W/S ratio of MAL-6 in cortical synaptosomal membranes from SAMP8 mice that had been injected for 14 days with the free radical scavenger PBN before death is higher than that in cortical synaptosomal membranes from those SAMP8 mice injected with saline (P < 0.001), which is consistent with less oxidative stress in PBN-treated age-accelerated mice. However, age-resistant SAMR1 mice injected with the same dose of PBN for 14 days showed no significant change in the W/S ratio relative to saline-injected SAMR1 mice (data not shown), consistent with the notion that less oxidative stress occurs in untreated SAMR1 mice compared with SAMP8 mice.
Figure 3.
Data represent the average W/S ratio of MAL-6 covalently bound to proteins in cortical synaptosomal membranes from SAMP8 mice previously injected with saline or PBN (30 mg/kg) for 14 days. Error bars indicate SEM for five animals each. ∗, P < 0.001.
DISCUSSION
Many groups have studied the status of learning, memory, and other behaviors of the SAMP8 age-accelerated mouse relative to the SAMR1 age-resistant mouse. Using aversive and appetitive training tests, Flood et al. (26) reported that SAMP8 mice exhibited an early onset of impaired learning relative to SAMR1 mice. Ohta et al. (28) analyzed the SAMP8 and SAMR1 mice using Sidman active-avoidance learning. These researchers concluded that SAMP8 mice have a deficit in reference memory rather than working memory. By studying passive avoidance, Yagi et al. (27) determined that in the SAMP8 mouse the deterioration in memory and learning is caused by deficits in acquisition. Based on the current studies, we suggest that these deficits in memory and learning may be related to altered membrane protein structure due to free radical oxidative stress. Recently, Forster et al. (39) showed in 22-month-old C57 mice both loss of cognitive and motor skills and increased brain protein oxidation, consistent with the results presented in the current research.
Nomura et al. (40) compared several biochemical parameters of SAMP8 and SAMR1 mice that reflect various degrees of senescence. In all instances, the SAMP8 mouse exhibited rapid biochemical changes compared with those that would be considered normal aging. Consistent with our finding of free radical-associated brain membrane damage reported here, these scientists reported that levels of malondialdehyde, an end-product of free radical reactions, were higher in SAMP8 brain than in the SAMR1 brain (40). Likewise, chronic administration of the free radical scavenger PBN was reported by Edamatsu et al. (41) to increase the life span of SAMP8 mice, consistent with a free radical involvement in aging and our results.
There is considerable literature to suggest that free radical scavengers can be used to prevent free radical damage in a variety of systems. Consistent with the present results suggesting that free radical-induced brain membrane protein damage in the SAMP8 mouse can be ameliorated by PBN, there are similar studies that suggest that PBN can prevent damage induced by aging. When PBN (32 mg/kg) i.p. was injected into aged gerbils twice a day for 14 days, the amount of oxidized brain proteins decreased nearly to that observed in adult gerbils (42). Likewise, after chronic PBN treatment the GS and neutral protease activity of the older gerbils returned to that observed in the younger animals (42). This report was questioned (43), but others have confirmed the original findings (44). In rats, too, chronic PBN administration is reported to improve cognitive function (45). As noted above, SAMP8 mice injected daily with 30 mg/kg PBN exhibited a remarkable extension of the mean life span: the mice injected with PBN experienced about a 50% increase in life span (41). Consonant with our results on aging, PBN is reported to prevent free radical-induced damage to cortical membranes in a gerbil stroke model (4) and in the hyperoxia model of aging (12).
Although the defects associated with progeria, Werner syndrome and other accelerated-aging conditions are not well understood, it is interesting to speculate whether the SAMP8 is a useful model of these diseases. Fibroblasts obtained from both progeria and Werner patients demonstrate increased carbonyl content in tissue culture (18, 20). The accumulation of cytosolic protein carbonyl is greatly accelerated across the life span compared with normal controls. This is the same as we have reported for SAMP8 vs. SAMR1 mice. The observed beneficial effects of PBN in SAMP8 mice reported here offer the possibility of developing antioxidant approaches to treating these and other diseases of aging.
The results reported in this study strongly suggest that SAMP8 and SAMR1 mice differ in baseline levels of oxidation in both neuronal cell membrane and cytosolic protein, consistent with free radical-induced damage in aging. That PBN is effective at protecting synaptosomal membrane protein structure from oxidative stress in accelerated senescence (this study) and prolongs the life span of these mice (41) suggests that further study of PBN or similar compounds is warranted; such studies conceivably may lead to valuable insight into how free radical damage in aging and age-related neurodegenerative disorders may be slowed. Investigations along this line are underway currently in our laboratory.
Acknowledgments
We thank Centaur Pharmaceuticals for the generous gift of PBN. This work was supported in part by grants from the National Institutes of Health (AG-10836 and AG-05119).
Footnotes
Abbreviations: SAMP8, senescence accelerated-prone mouse; SAMR1, senescence accelerated-resistant mouse; GS, glutamine synthetase; PBN, N-tert-butyl-α-phenylnitrone; MAL-6, 2,2,6,6,-tetramethyl-4-maleimidopiperidine-1-oxyl; W/S ratio, MI = +1 low-field weakly immobilized line/MI = +1 low-field strongly immobilized line ratio.
References
- 1.Hensley K, Howard B, Carney J, Butterfield D A. Biochim Biophys Acta. 1995;1270:203–206. doi: 10.1016/0925-4439(95)00043-4. [DOI] [PubMed] [Google Scholar]
- 2.Butterfield D A, Hensley K, Harris M, Mattson M, Carney J. Biochem Biophys Res Commun. 1994;200:710–715. doi: 10.1006/bbrc.1994.1508. [DOI] [PubMed] [Google Scholar]
- 3.Hensley K, Carney J, Hall N, Shaw W, Butterfield D A. Free Radical Biol Med. 1994;17:321–331. doi: 10.1016/0891-5849(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 4.Hall N, Carney J, Cheng M, Butterfield D A. Neuroscience. 1995;69:591–600. doi: 10.1016/0306-4522(95)00289-u. [DOI] [PubMed] [Google Scholar]
- 5.Hall N, Carney J, Cheng M, Butterfield D A. Neuroscience. 1995;64:81–89. doi: 10.1016/0306-4522(94)00385-i. [DOI] [PubMed] [Google Scholar]
- 6.Hall N, Dempsey R, Carney J, Donaldson D, Butterfield D A. Neurochem Res. 1995;20:1161–1169. doi: 10.1007/BF00995379. [DOI] [PubMed] [Google Scholar]
- 7.Hall, N. C., Carney, J. M., Plante, O. J., Cheng, M. & Butterfield, D. A. (1997) Neuroscience, in press. [DOI] [PubMed]
- 8.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita P, Wu J F, Carney J M, Lovell M, Markesbery W, Butterfield D A. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 9.Butterfield D A, Hensley K, Martin L, Carney J. Life Sci. 1996;58:217–228. doi: 10.1016/0024-3205(95)02279-1. [DOI] [PubMed] [Google Scholar]
- 10.Butterfield D A. In: Reactive Oxygen Species In Biological Systems: An Interdisciplinary Approach. Gilbert D L, Colton C A, editors. New York: Plenum; 1997. in press. [Google Scholar]
- 11.Butterfield D A. In: Molecular Models of Dementia. Wasco W, Tanzi R, editors. Totowa, NJ: Humana; 1996. pp. 145–167. [Google Scholar]
- 12.Howard B J, Yatin S, Allen K, Carney J, Butterfield D A. J Neurochem. 1996;67:2045–2050. doi: 10.1046/j.1471-4159.1996.67052045.x. [DOI] [PubMed] [Google Scholar]
- 13.Hensley K, Mattson M, Aksenova M, Harris M, Wu J, Floyd R, Carney J, Butterfield D A. Proc Natl Acad Sci USA. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomiyama T, Shoji A, Kataoka K-I, Suwa Y, Asano S, Kaneko H, Endo N. J Biol Chem. 1996;271:6839–6844. doi: 10.1074/jbc.271.12.6839. [DOI] [PubMed] [Google Scholar]
- 15.Harris M, Hensley K, Butterfield D A, Leedle R, Carney J. Exp Neurol. 1995;131:193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 16.Butterfield D A. Alzheimer’s Dis Rev. 1996;1:68–70. [Google Scholar]
- 17.Hensley K, Butterfield D A, Hall N, Cole P, Subramaniam R, Mark R, Mattson M P, Markesbery W R, Harris M E, Aksenov M, Aksenova M, Wu J F, Carney J M. Ann NY Acad Sci. 1996;786:120–134. doi: 10.1111/j.1749-6632.1996.tb39057.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferrante R, Kowall N, Cippollon P, Storey E, Beal M F. Exp Neurol. 1993;119:46–47. doi: 10.1006/exnr.1993.1006. [DOI] [PubMed] [Google Scholar]
- 19.Stadtman E. Science. 1992;257:1120–1124. [Google Scholar]
- 20.Oliver C, Ahn B, Moerman E, Goldstein S, Stadtman E. J Biol Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- 21.Harman D. Ann NY Acad Sci. 1994;717:1–15. doi: 10.1111/j.1749-6632.1994.tb12069.x. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell B, Gutteridge J M C. Methods Enzymol. 1984;105:22–36. doi: 10.1016/s0076-6879(84)05007-2. [DOI] [PubMed] [Google Scholar]
- 23.Gutteridge J M. Free Radical Res Commun. 1993;19:141–148. doi: 10.3109/10715769309111598. [DOI] [PubMed] [Google Scholar]
- 24.Evans D A, Funkenstein H H, Albert M S. J Am Med Assoc. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 25.Takeda T, Hosokawa M, Takeshita S, Irino M. Mech Ageing Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 26.Flood J, Morley J, Reginna M. Neurobiol Aging. 1993;14:159–166. doi: 10.1016/0197-4580(93)90092-p. [DOI] [PubMed] [Google Scholar]
- 27.Yagi H, Katoh T, Agiguti I, Takeda T. Brain Res. 1988;474:86–93. doi: 10.1016/0006-8993(88)90671-3. [DOI] [PubMed] [Google Scholar]
- 28.Ohta A, Hirano T, Yagi H, Tanaka S. Brain Res. 1989;498:195–198. doi: 10.1016/0006-8993(89)90421-6. [DOI] [PubMed] [Google Scholar]
- 29.Butterfield D A. Biol Magn Res. 1982;4:1–78. [Google Scholar]
- 30.Carney J, Carney A. Life Sci. 1994;55:2097–2103. doi: 10.1016/0024-3205(94)00390-4. [DOI] [PubMed] [Google Scholar]
- 31.Smith C D, Carney J, Tatsumo T, Stadtman E, Floyd R, Markesbery W. Ann NY Acad Sci. 1992;663:110–119. doi: 10.1111/j.1749-6632.1992.tb38654.x. [DOI] [PubMed] [Google Scholar]
- 32.Lowry O, Rosenbrough N, Farr A, Randall R. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 33.Umhauer S, Isbell D, Butterfield D A. Anal Lett. 1992;25:1201–1215. [Google Scholar]
- 34.Rowe W, Remzio R, Wellner V, Meister A. Methods Enzymol. 1970;17:900–910. [Google Scholar]
- 35.Miller R, Haderberg R, Gersham H. Proc Natl Acad Sci USA. 1978;75:1418–1422. doi: 10.1073/pnas.75.3.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trad C, Butterfield D A. Toxicol Lett. 1994;73:145–155. doi: 10.1016/0378-4274(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 37.Bellary S, Anderson K, Arden W, Butterfield D A. Life Sci. 1994;56:91–98. doi: 10.1016/0024-3205(94)00418-r. [DOI] [PubMed] [Google Scholar]
- 38.Starke-Reed P, Oliver C. Arch Biochem Biophys. 1989;275:559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- 39.Forster M, Dubey A, Dawson K, Stutts W, Lal H, Sohal R. Proc Natl Acad Sci USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura Y, Wang B, Qi S, Tsuneo N, Kaneko S. Exp Gerontol. 1989;24:49–55. doi: 10.1016/0531-5565(89)90034-x. [DOI] [PubMed] [Google Scholar]
- 41.Edamatsu R, Mori A, Packer L. Biochem Biophys Res Commun. 1995;211:847–849. doi: 10.1006/bbrc.1995.1889. [DOI] [PubMed] [Google Scholar]
- 42.Carney J, Starke-Reed P, Oliver C, Landum R, Cheng M, Wu J, Floyd R. Proc Natl Acad Sci USA. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao G, Cutler R G. Arch Biochem Biophys. 1995;320:106–114. doi: 10.1006/abbi.1995.1347. [DOI] [PubMed] [Google Scholar]
- 44.Dubey A, Forster M J, Sohal R S. Arch Biochem Biophys. 1995;324:249–254. doi: 10.1006/abbi.1995.0037. [DOI] [PubMed] [Google Scholar]
- 45.Socci D J, Crandall B M, Arendash G W. Brain Res. 1995;693:88–94. doi: 10.1016/0006-8993(95)00707-w. [DOI] [PubMed] [Google Scholar]