Abstract
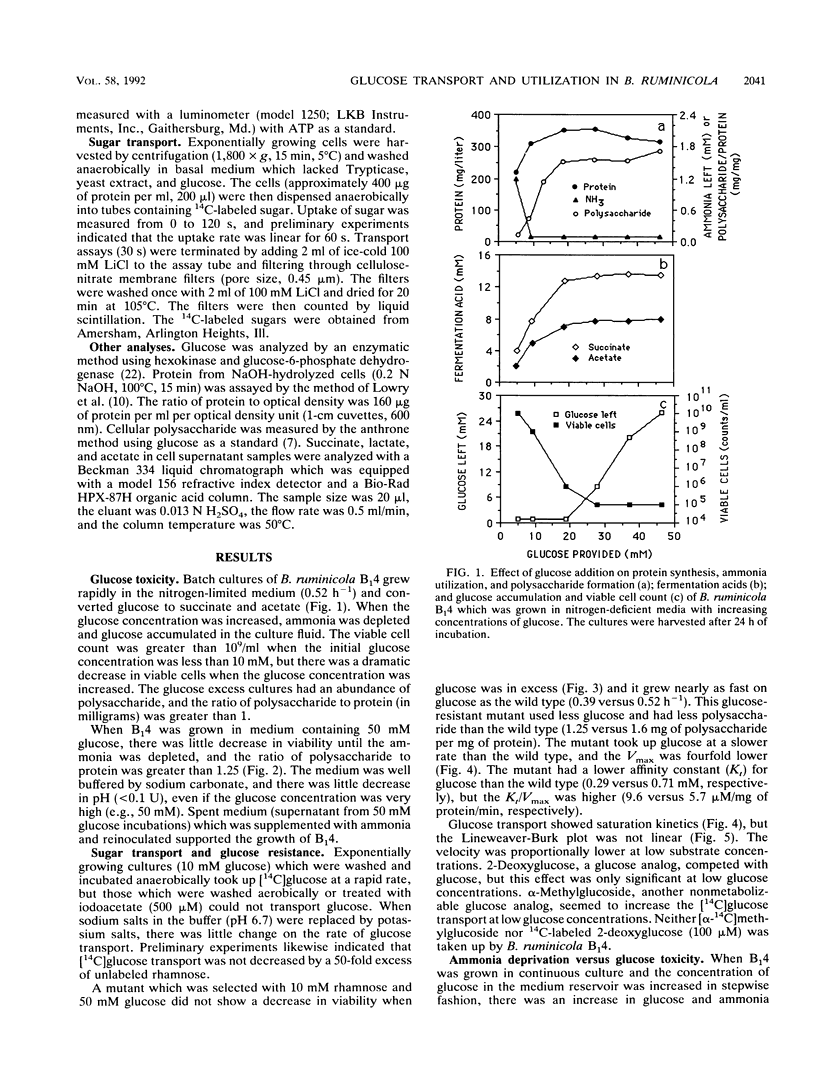
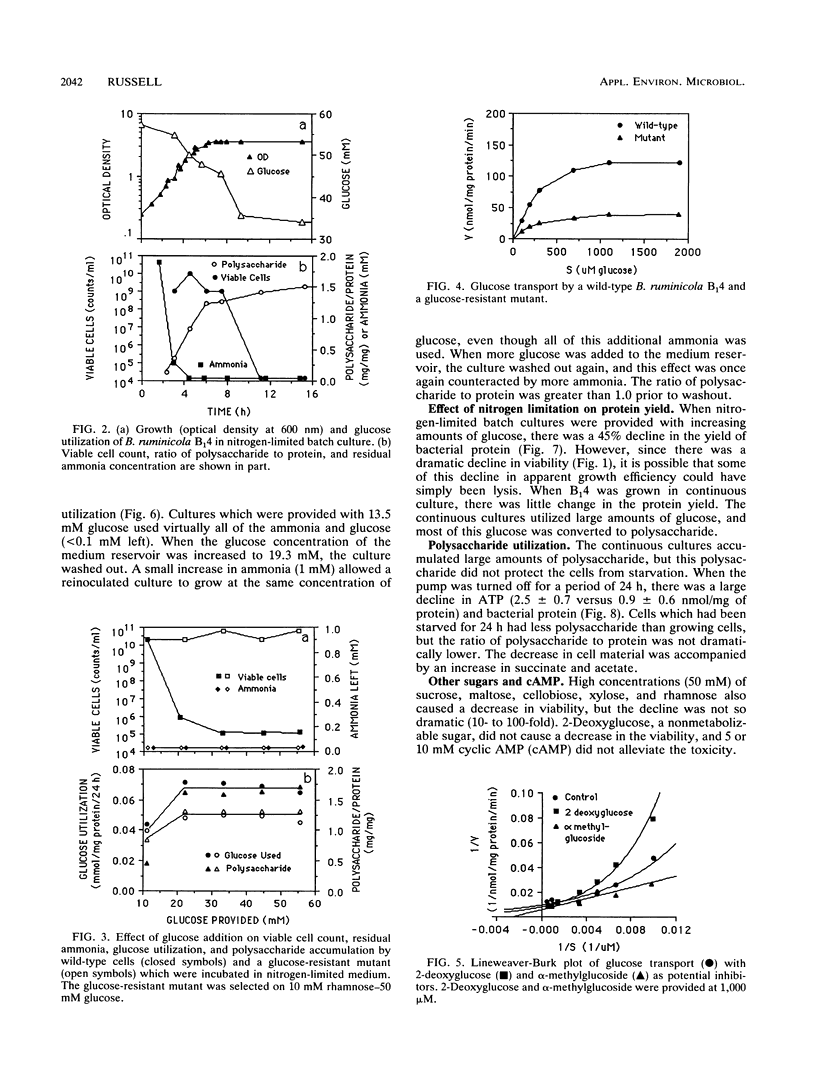
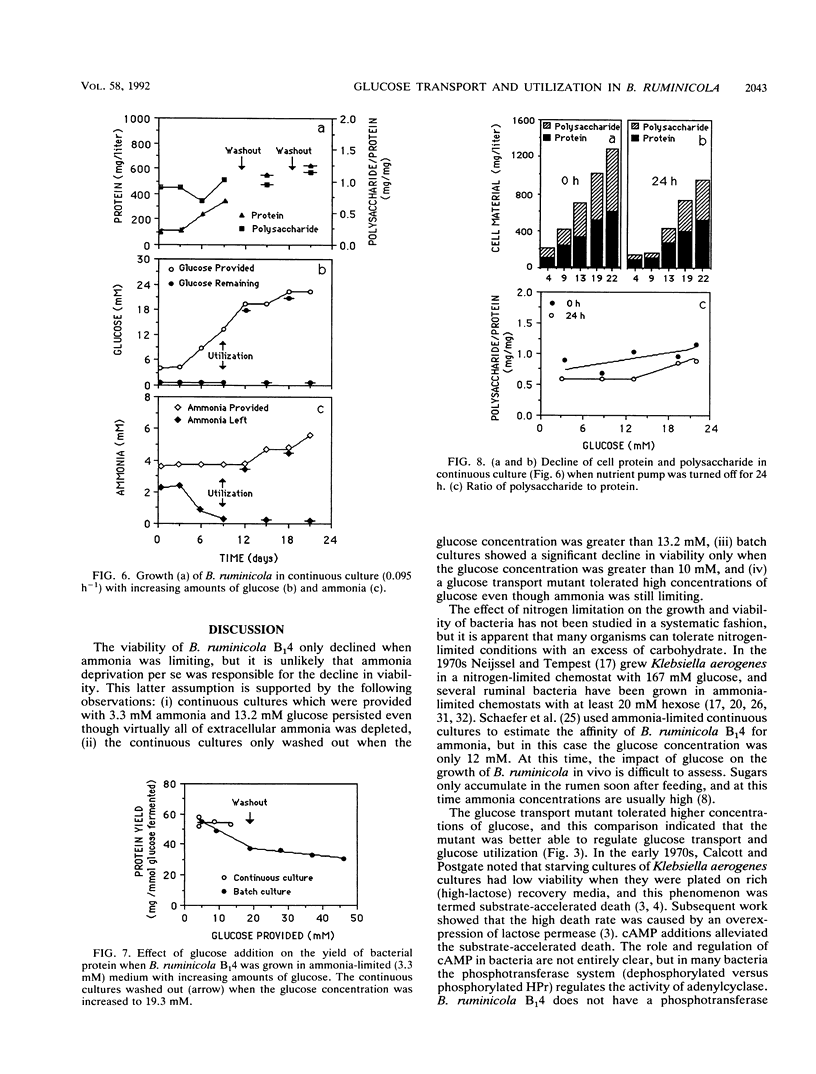
Ammonia-limited (3.5 mM ammonia) cultures of Bacteroides ruminicola B(1)4 had a high number of viable cells (greater than 10(9)/ml), but only when the concentration of glucose was not too high (10 mM or less). When the glucose concentration was increased from 10 to 50 mM, there was a marked decrease in viability (10(5)-fold or greater). Because there was little decline in pH and only a small increase in succinate and acetate as the glucose concentration was increased, it did not appear that end products were killing the cells. This conclusion was supported by the observation that reinoculated cultures grew in the spent medium which had been supplemented with ammonia. Unlabeled rhamnose did not inhibit [14C]-glucose uptake, and cultures which were selected with a low concentration of rhamnose tolerated high concentrations of glucose (50 mM). The glucose-resistant mutant transported glucose at a lower rate than the wild type, and the Vmax of glucose transport was fourfold lower. The wild type stored much more polysaccharide than the glucose-resistant mutant, but it is not clear if polysaccharide accumulation per se is responsible for the glucose toxicity. These results indicated that B. ruminicola B(1)4 is unable to regulate glucose transport and utilization when growth is limited by ammonia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buurman E. T., Teixeira de Mattos M. J., Neijssel O. M. Futile cycling of ammonium ions via the high affinity potassium uptake system (Kdp) of Escherichia coli. Arch Microbiol. 1991;155(4):391–395. doi: 10.1007/BF00243460. [DOI] [PubMed] [Google Scholar]
- Calcott P. H., Postgate J. R. On substrate-accelerated death in Klebsiella aerogenes. J Gen Microbiol. 1972 Apr;70(1):115–122. doi: 10.1099/00221287-70-1-115. [DOI] [PubMed] [Google Scholar]
- Calcott P. H., Postgate J. R. The effects of beta-galactosidase activity and cyclic AMP on lactose-accelerated death. J Gen Microbiol. 1974 Nov;85(1):85–90. doi: 10.1099/00221287-85-1-85. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. M., Russell J. B. Effect of pH and Monensin on Glucose Transport by Fibrobacter succinogenes, a Cellulolytic Ruminal Bacterium. Appl Environ Microbiol. 1992 Apr;58(4):1115–1120. doi: 10.1128/aem.58.4.1115-1120.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K. A., Preziosi M. C., Caldwell D. R. Some effects of uncouplers and inhibitors on growth and electron transport in rumen bacteria. J Bacteriol. 1979 Aug;139(2):384–392. doi: 10.1128/jb.139.2.384-392.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett M. R., Mountfort D. O., Turner K. W., Roberton A. M. Metabolism and growth yields in Bacteroides ruminicola strain b14. Appl Environ Microbiol. 1976 Aug;32(2):274–283. doi: 10.1128/aem.32.2.274-283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J., Probst I., Gottschalk G. Evidence for cytochrome involvement in fumarate reduction and adenosine 5'-triphosphate synthesis by Bacteroides fragilis grown in the presence of hemin. J Bacteriol. 1975 Aug;123(2):436–442. doi: 10.1128/jb.123.2.436-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Phosphoenolpyruvate-dependent phosphorylation of hexoses by ruminal bacteria: evidence for the phosphotransferase transport system. Appl Environ Microbiol. 1986 Dec;52(6):1348–1352. doi: 10.1128/aem.52.6.1348-1352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Fermentations by saccharolytic intestinal bacteria. Am J Clin Nutr. 1979 Jan;32(1):164–172. doi: 10.1093/ajcn/32.1.164. [DOI] [PubMed] [Google Scholar]
- Mink R. W., Hespell R. B. Long-term nutrient starvation of continuously cultured (glucose-limited) Selenomonas ruminantium. J Bacteriol. 1981 Nov;148(2):541–550. doi: 10.1128/jb.148.2.541-550.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient chemostat culture. Arch Microbiol. 1976 Mar 19;107(2):215–221. doi: 10.1007/BF00446843. [DOI] [PubMed] [Google Scholar]
- Patterson J. A., Hespell R. B. Glutamine synthetase activity in the ruminal bacterium Succinivibrio dextrinosolvens. Appl Environ Microbiol. 1985 Oct;50(4):1014–1020. doi: 10.1128/aem.50.4.1014-1020.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman K. A., Lakshmanan S., Bryant M. P. Oligopeptide uptake by Bacteroides ruminicola. J Bacteriol. 1967 May;93(5):1499–1508. doi: 10.1128/jb.93.5.1499-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of maintenance energy expenditures and growth yields among several rumen bacteria grown on continuous culture. Appl Environ Microbiol. 1979 Mar;37(3):537–543. doi: 10.1128/aem.37.3.537-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. Fermentation of Peptides by Bacteroides ruminicola B(1)4. Appl Environ Microbiol. 1983 May;45(5):1566–1574. doi: 10.1128/aem.45.5.1566-1574.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. ATPase-dependent energy spilling by the ruminal bacterium, Streptococcus bovis. Arch Microbiol. 1990;153(4):378–383. doi: 10.1007/BF00249009. [DOI] [PubMed] [Google Scholar]
- Schaefer D. M., Davis C. L., Bryant M. P. Ammonia saturation constants for predominant species of rumen bacteria. J Dairy Sci. 1980 Aug;63(8):1248–1263. doi: 10.3168/jds.S0022-0302(80)83076-1. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Hespell R. B., Bryant M. P. Ammonia assimilation and glutamate formation in the anaerobe Selenomonas ruminantium. J Bacteriol. 1980 Feb;141(2):593–602. doi: 10.1128/jb.141.2.593-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- WHITE D. C., BRYANT M. P., CALDWELL D. R. Cytochromelinked fermentation in Bacteroides ruminicola. J Bacteriol. 1962 Oct;84:822–828. doi: 10.1128/jb.84.4.822-828.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachenheim D. E., Hespell R. B. Responses of Ruminococcus flavefaciens, a Ruminal Cellulolytic Species, to Nutrient Starvation. Appl Environ Microbiol. 1985 Dec;50(6):1361–1367. doi: 10.1128/aem.50.6.1361-1367.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J. Control of lactate production by Selenomonas ruminantium: homotropic activation of lactate dehydrogenase by pyruvate. J Gen Microbiol. 1978 Jul;107(1):45–52. doi: 10.1099/00221287-107-1-45. [DOI] [PubMed] [Google Scholar]


