Abstract
Reperfusion of the ischemic myocardium results in the generation of oxygen-derived free radicals, NO, and presumably peroxynitrite. These, in turn, may cause strand breaks in DNA, which activate the nuclear enzyme poly(ADP ribose) synthetase (PARS). This results in a rapid depletion of intracellular NAD and ATP. When this reaction is excessive, there is ultimately cell death. Here we demonstrate that 3-aminobenzamide (and several other, chemically distinct, inhibitors of PARS activity) reduces the infarct size caused by ischemia and reperfusion of the heart or skeletal muscle of the rabbit. Inhibition of PARS activity also attenuates the myocardial dysfunction caused by global ischemia and reperfusion in the isolated, perfused heart of the rabbit. In skeletal muscle, inhibition of the activity of neuronal NO synthase reduces infarct size, indicating that the formation of NO contributes to the activation of PARS there. There is no significant neuronal NO synthase activity in the heart, and hence NO synthase inhibitors did not reduce myocardial infarct size. Thus, activation of PARS contributes to the cell death caused by ischemia–reperfusion, and PARS inhibitors may constitute a novel therapy for ischemia–reperfusion injury.
Poly(ADP ribose) synthetase (PARS; EC 2.4.2.30) is a chromatin-bound enzyme, which plays a physiological role in the repair of strand breaks in DNA (1). PARS is located in the nuclei of cells of various tissues, including the heart and skeletal muscle (2). When activated by strand breaks in DNA, PARS catalyzes the transfer of ADP ribose moieties from NAD with the concomitant formation of nicotinamide. This results in a substantial depletion of NAD. Nicotinamide, which inhibits PARS activity by negative feedback, can be recycled to NAD in a reaction that consumes ATP. As NAD is essential to mitochondrial electron transport, depletion of NAD rapidly leads to a fall in ATP, and ultimately cell death (3–5). Radicals including superoxide anions, hydrogen peroxide or hydroxyl radicals (3–5), and NO or peroxynitrite (6–8) cause the breakage of DNA strands and activation of PARS. Inhibitors of PARS activity attenuate the fall in NAD and ATP and improve survival of cultured cells (e.g., fibroblasts, endothelial cells, and smooth muscle cells) exposed to oxygen-derived free radicals (3–5, 9), NO (6–8), or peroxynitrite (8). The formation of radicals contributes to the “reperfusion injury” of previously ischemic organs, including the heart (10) and the skeletal muscle (11). Here we demonstrate that several inhibitors of PARS activity reduce the infarct size caused by ischemia–reperfusion of the heart and skeletal muscle.
MATERIALS AND METHODS
Determination of Myocardial Infarct Size (in Vivo).
Male New Zealand white rabbits were premedicated [Hypnorm (Janssen, Saunderton, U.K.), 0.1 ml·kg−1 i.m.; containing 0.315 mg·ml−1 fentanyl citrate and 10 mg·ml−1 fluanisone] and anesthetized (pentobarbitone, 20 mg·kg−1 i.v.). Following tracheotomy and ventilation (36–40 strokes·min−1, tidal volume: 18–20 ml), a thoracotomy was performed and a suture placed around the first antero-lateral branch of the left coronary artery (LAL; ref. 12). Hemodynamic parameters [left ventricular systolic pressure (LVSP), mean arterial blood pressure (MAP), heart rate, and pressure rate index (PRI), an indicator of myocardial oxygen consumption] were measured. The LAL was occluded (45 min) and opened to allow reperfusion (2 h). Area at risk (AAR) was determined by staining of the perfused myocardium (Evans blue dye), and infarct size was determined by staining of the AAR with p-nitro-blue tetrazolium (NBT, 0.5 mg·ml−1, 20 min at 37°C) as previously described (12). The AAR (all animal groups) ranged from 33 ± 4% to 40 ± 4% (P > 0.05, unpaired Student’s t test).
Determination of Left Ventricular Function (Isolated Heart).
Rabbits were anesthetized and ventilated (see above) and they received heparin (1000 units·kg−1). After thoracotomy, the heart was excised and perfused (according to Langendorff) retrogradely at constant flow (40 ml·min−1, stimulated at 180 beats·min−1) with modified Krebs’–Henseleit solution, composed of 118.0 mM NaCl, 25.0 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10.0 mM d-glucose, and 1.8 mM CaCl2 gassed at 37°C with 95% O2/5% CO2. Coronary perfusion pressure (CPP), left ventricular developed pressure (LVDP), and left ventricular end diastolic pressure (LVEDP) were measured. Following no-flow global ischemia (30 min) and reperfusion (2 h), infarct size was determined by staining of the left ventricle with NBT.
Determination of Skeletal Muscle Necrosis (in Vivo).
Rabbits were premedicated, anesthetized, ventilated (as above), and instrumented for the measurement of MAP and heart rate. After a midline laparotomy, the bifurcation of the aorta was isolated. Thirty minutes later, the distal aorta was occluded (4 h) and reperfused (3 h). The left and right gracilis muscles were excised in toto and percent necrosis was determined by staining of muscle slices with NBT and weighing stained (alive) and unstained (infarcted) tissue.
Determination of Inducible NO Synthase (iNOS) Protein Expression.
Western blot (immunoblot) analysis for iNOS protein was performed as previously described (13). The gracilis muscle was homogenized on ice in an extraction buffer (pH 7.4) consisting of 50 mM Tris·HCl, 10 mM EDTA, 1% (vol/vol) Triton X-100, and protease inhibitors (50 μM pepstatin A, 0.2 mM leupeptin, and 1 mM phenylmethylsulfonyl fluoride). The cell extract was boiled for 10 min with gel-loading buffer [20 mM Tris/2 mM EDTA/2% (wt/vol) SDS/20% (vol/vol) glycerol/10% (vol/vol) 2-mercaptoethanol/2 mg·ml−1 bromophenol blue, pH 6.8] in a ratio of 1:1 (vol/vol). Samples were centrifuged at 10,000 × g for 2 min before being loaded onto gradient gels (7.5% SDS gel) and subjected to electrophoresis (1 h at 150 V). The separated proteins were transferred to nitrocellulose (Bio-Rad; 1 h at 200 V). After transfer to nitrocellulose by electrophoresis, the membranes were primed with a polyclonal antibody raised to macrophage iNOS developed in rabbits. (14). The blots were then incubated with anti-rabbit IgG linked to horseradish peroxidase. All antibodies were used at a 1:2000 dilution. Subsequently, the Western blots were developed with diaminobenzamine used as a substrate. iNOS protein was not detected in skeletal muscle homogenate obtained from gracilis muscle after 4 h of ischemia and 3 h of reperfusion. The procedure used detected the expression of iNOS protein in tissue homogenates of rats challenged with endotoxin for 6 h (positive control; ref. 13).
Drugs.
With the exception of 4-amino-1,8-naphthalimide and 1,5-dihydroxyisoquinoline (10% dimethyl sulfoxide; DMSO), all PARS inhibitors were dissolved in saline. The NO synthase (NOS) inhibitor 7-nitroindazole (7-NI) was dissolved in 10% DMSO in arachis oil, while all other NOS inhibitors were dissolved in saline. Injection of the vehicle containing DMSO did not result in a significant reduction in infarct size in heart (53 ± 3%, P > 0.05, n = 5) or skeletal muscle (49 ± 6%, P > 0.05, n = 6). Similarly, the vehicle containing DMSO and arachis oil did not affect the degree of necrosis caused by ischemia and reperfusion in skeletal muscle (41 ± 10%, P > 0.05, n = 6). None of the agents (PARS inhibitors or NOS inhibitors) caused significant alterations in any of the hemodynamic parameters measured. All the inhibitors of PARS used in this study have been well characterized in terms of their ability to inhibit PARS activity in various different cells and tissues.
Materials.
The PARS inhibitors 4-amino-1,8-naphthalimide and 1,5-dihydroxyisoquinoline were obtained from Aldrich. 3-aminobenzoic acid was from Fluka. All other inhibitors of PARS or NOS activity as well as all other reagents were from Sigma.
Statistical Analysis.
All data in tables, text, and figures are expressed as mean ± SEM. Statistical comparisons between groups were made by ANOVA followed by Bonferoni’s test for multiple comparisons. P < 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
Inhibitors of PARS Activity Reduce Infarct Size in a Rabbit Model of Regional Myocardial Ischemia and Reperfusion.
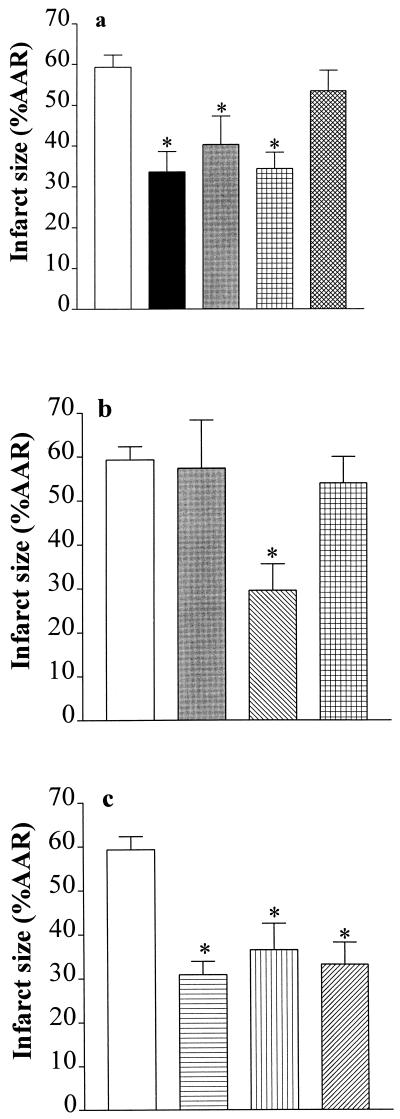
Administration of the PARS inhibitor 3-aminobenzamide [3-AB, 10 mg·kg−1 intraarterially (i.a.); refs. 12 and 13] 1 min before the occlusion (45 min) and before reperfusion (2 h) of the first antero-lateral branch of the LAL caused a 43% reduction in infarct size, without affecting hemodynamic parameters. Single injections of 3-AB either before occlusion or reperfusion caused similar reductions in infarct size (32% or 42%; Fig. 1a). Thus, activation of PARS during reperfusion contributes to the extension of infarct size in the rabbit. Although 3-AB is a selective inhibitor of PARS activity (15, 16), it may be argued that the cardioprotection afforded by 3-AB is due to nonspecific effects. This is, however, unlikely, for 3-aminobenzoic acid (10 mg·kg−1 i.a. at 1 min before reperfusion), an analogue of 3-AB, which does not inhibit PARS activity (negative control; refs. 15 and 16), did not cause a reduction in infarct size (Fig. 1a). Moreover, 3-AB prevents the fall in NAD and cell death caused by oxygen-derived free radicals or NO in murine islet cells (wild-type), but not in mutant cells, in which the PARS gene (“knock-out”) had been inactivated (6). Our hypothesis that the activation of PARS contributes to injury measured after reperfusion of ischemic tissue is further supported by our findings that nicotinamide (negative feedback, endogenous PARS inhibitor) and three other chemically distinct inhibitors of PARS activity also reduce infarct size, but do not cause hemodynamic effects. For instance, administration (before reperfusion) of nicotinamide (20 mg·kg−1 i.a.) caused a 50% reduction of infarct size (Fig. 1b), while the PARS inhibitors 4-aminobenzamide (10 mg·kg−1 i.a.), 4-amino-1,8-naphthalimide (refs. 15 and 16; 1 mg·kg−1 i.a.) or 1,5-dihydroxyisoquinoline (refs. 7, 15, and 16; 1 mg·kg−1 i.a.) reduced infarct size by 38–48% (Fig. 1c). As nicotinamide enhances the intracellular levels of NAD, the cardioprotective effects of nicotinamide may be secondary to an increase in the levels of NAD and subsequently ATP. This is, however, unlikely, for nicotinic acid also enhances the intracellular levels of NAD, but without forming nicotinamide as an intermediate. Nicotinic acid, however, did not reduce myocardial infarct size (when given at the same dose as nicotinamide; Fig. 1b). As nicotinamide, but not nicotinic acid (15, 16), inhibits the activity of PARS, these results support our hypothesis that the cardioprotective effects of nicotinamide are, indeed, due to inhibition of PARS activity and not due to an augmentation by nicotinamide of the intracellular levels of NAD.
Figure 1.
Inhibitors of PARS activity reduce myocardial infarct size in the rabbit. (a) When compared with controls (open column; n = 9), 3-AB (10 mg·kg−1 i.a.) reduced infarct size when given (i) 1 min before LAL occlusion and 1 min before reperfusion (black column, n = 9); (ii) 1 min before occlusion (gray column, n = 6); or (iii) 1 min before reperfusion (grid-patterned column, n = 7). In contrast, 3-aminobenzoic acid (10 mg·kg−1 i.a; given 1 min before reperfusion; cross-hatched column, n = 5) did not reduce infarct size. (b) When compared with controls (open column; n = 9), i.a. injection 1 min before reperfusion of nicotinamide (20 mg·kg−1 i.a., hatched column, n = 5), but not of 10 mg·kg−1 (light gray column, n = 3) caused a reduction in infarct size. Furthermore, nicotinic acid (20 mg·kg−1 i.a., grid-patterned column, n = 5) had no effect on infarct size. (c) The PARS inhibitors 4-aminobenzamide (10 mg·kg−1, horizontal striped column, n = 5), 4-amino-1,8-naphthalimide (1 mg·kg−1, vertical striped column, n = 5), and 1,5-dihydroxyisoquinoline (1 mg·kg−1, hatched column, n = 5) also caused reductions in infarct size. ∗, P < 0.05 compared with control (ANOVA followed by Bonferoni’s test).
Inhibitors of PARS Activity Attenuate the Myocardial Dysfunction Caused by Global Myocardial Ischemia and Reperfusion in the Isolated Rabbit Heart.
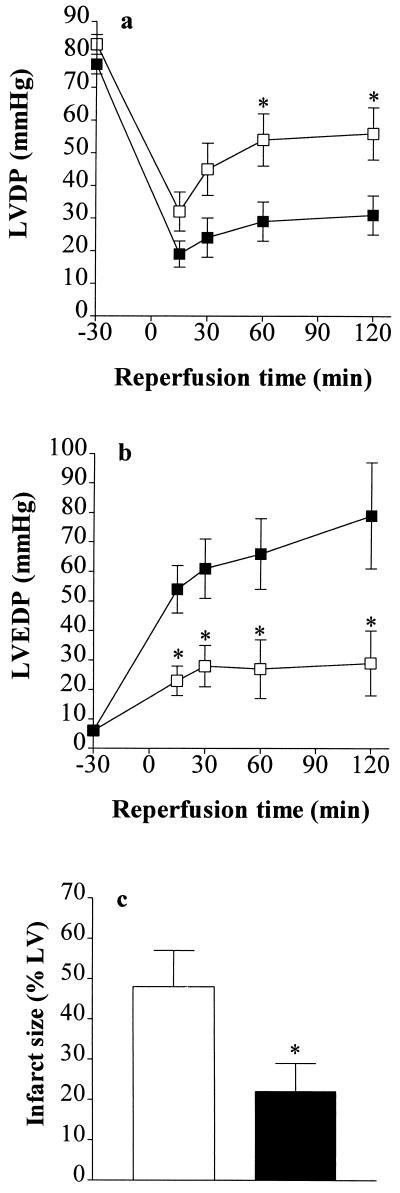
To elucidate whether inhibition of PARS activity also attenuates the myocardial contractile dysfunction caused by ischemia–reperfusion, we investigated the effects of 3-AB on the alterations in cardiac function caused by global myocardial ischemia and reperfusion in the isolated heart of the rabbit. Global ischemia (30 min) and reperfusion (2 h) resulted in a 60% impairment in left ventricular developed pressure (Fig. 2a) and a substantial rise in left ventricular end diastolic pressure (Fig. 2b). Reperfusion of the heart with buffer containing 3-AB (100 μM for the first 60 min of the reperfusion period) largely attenuated this contractile dysfunction (Fig. 2 a and b) and also reduced infarct size (Fig. 2c). Thus, (i) 3-AB interferes with a process leading to the death of cardiomyocytes during reperfusion, and (ii) the mechanism of its cardioprotection is independent of alterations in myocardial blood flow or of the inhibition of the function of platelets or neutrophils.
Figure 2.
The PARS inhibitor 3-AB attenuates the contractile dysfunction and reduces infarct size of hearts subjected to global ischemia and reperfusion. Thirty minutes of global ischemia followed by 2 h of reperfusion (▪, vehicle control, n = 7) resulted in (a) a substantial impairment of left ventricular developed pressure (LVDP) and (b) a rise in left ventricular end diastolic pressure (LVEDP) in the isolated, perfused heart of the rabbit. Infusion (into the perfusion buffer) of 3-AB (□, final concentration 100 μM, n = 6) resulted in a significant improvement in the recovery of contractile function and attenuated the rise in LVEDP. ∗, P < 0.05, ANOVA followed by Bonferoni’s test when compared with vehicle control. (c) When compared with control (open column, n = 7), 3-AB (solid column, n = 6) caused a significant reduction in infarct size.
Inhibitors of PARS Activity Reduce the Degree of Skeletal Muscle Necrosis Caused by Ischemia and Reperfusion of the Rabbit Hind Limb.
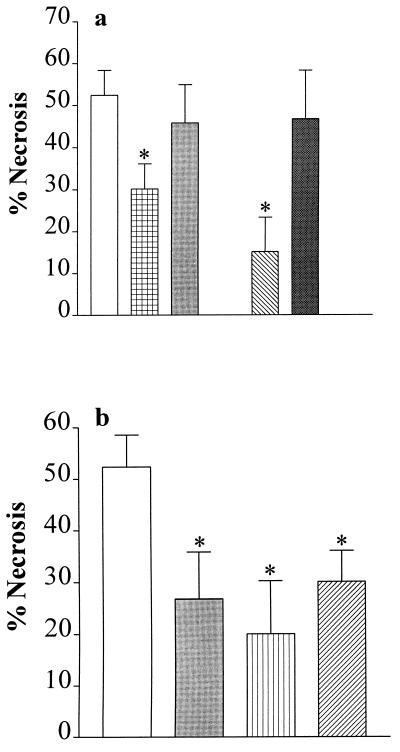
We have also investigated whether inhibition of PARS activity reduces the skeletal muscle necrosis caused by ischemia–reperfusion in the rabbit hind limb. Occlusion of the aorta (4 h) and reperfusion (3 h) resulted in an infarct size of 52% of the gracilis muscle (Fig. 3). Intravenous injection (1 min) before reperfusion of the PARS inhibitor 3-AB (10 mg·kg−1), but not of its inactive analogue 3-aminobenzoic acid (10 mg·kg−1), caused a significant reduction in the degree of skeletal muscle necrosis caused by ischemia–reperfusion of the hind limb (Fig. 3a). Similarly, nicotinamide, but not nicotinic acid (both at 20 mg·kg−1), reduced skeletal muscle necrosis when given 1 min before reperfusion (Fig. 3a). In addition, benzamide (1 mg·kg−1), 4-amino-1,8-naphthalimide (1 mg·kg−1), and 1,5-dihydroxyisoquinoline (1 mg·kg−1), which are potent inhibitors of PARS activity, resulted in a 40–50% reduction in infarct size in skeletal muscle (Fig. 3b). Thus, activation of PARS during reperfusion not only contributes to the death of cardiomyocytes, but also to the death of skeletal muscle myocytes challenged with ischemia–reperfusion.
Figure 3.
Inhibitors of PARS activity reduce the degree of skeletal muscle (gracilis) necrosis in a rabbit model of hind limb ischemia (4 h) and reperfusion (3 h). (a) When compared with vehicle (saline)-treated controls (open column; n = 18), the PARS inhibitor 3-AB (10 mg·kg−1 i.v., grid-patterned column, n = 16), but not its inactive analogue 3-aminobenzoic acid (10 mg·kg−1 i.v., gray column, n = 8), reduces skeletal muscle necrosis. Similarly, nicotinamide (20 mg·kg−1 i.v., hatched column, n = 6), but not nicotinic acid (20 mg·kg−1 i.v., dark gray column, n = 6), reduces skeletal muscle necrosis. (b) The PARS inhibitors benzamide (1 mg·kg−1 i.v., gray column, n = 10), 4-amino-1,8-naphthalimide (1 mg·kg−1 i.v., vertical striped column, n = 6), and 1,5-dihydroxyisoquinoline (1 mg·kg−1 i.v., hatched column, n = 10) also cause a significant reduction in the degree of skeletal muscle necrosis. ∗, P < 0.05 when compared with vehicle control by ANOVA followed by Bonferoni’s test.
Endogenous Nitric Oxide Contributes to the Activation of PARS Caused by Ischemia–Reperfusion of Skeletal Muscle, but Not of the Heart.
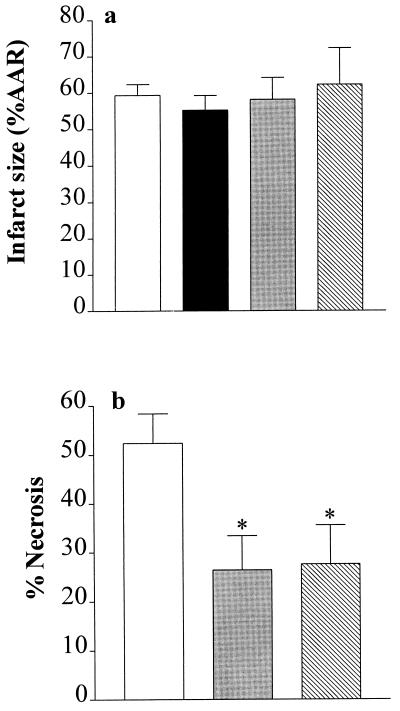
What, then, is responsible for the activation of PARS during reperfusion? In addition to reactive oxygen radicals, including hydrogen peroxide, hydroxyl radicals, and superoxide anions, the formation of NO and presumably peroxynitrite is enhanced during reperfusion of the previously ischemic myocardium. In cultured cells, all of these radicals cause DNA strand breaks and activate PARS (3, 8). Inhibition of NOS prevents the formation of NO and peroxynitrite (even in the presence of superoxide). To elucidate the potential contribution of NO or peroxynitrite to the activation of PARS, we have investigated the effects of various NOS inhibitors on infarct size. Administration (1 min before reperfusion) of the NOS inhibitors NG-methyl-l-arginine (3 mg·kg−1 i.a.; a nonselective inhibitor of all isoforms of NOS; ref. 17), 7-NI [30 mg·kg−1 i.p.; a selective inhibitor of neuronal NOS (nNOS; or NOS I); ref. 18], or aminoethyl-isothiourea (AE-ITU, 10 mg·kg−1 i.a.; a potent inhibitor of nNOS and iNOS (or NOS II); ref. 17] did not cause a reduction in myocardial infarct size (Fig. 4a). In contrast to the heart, inhibition of NOS activity with 7-NI (30 mg·kg−1 i.p.) or AE-ITU (10 mg·kg−1 i.v.) caused a substantial (≈50%) reduction in infarct size caused by ischemia–reperfusion in the gracilis muscle (Fig. 4b), without causing a significant increase in blood pressure (an indicator of inhibition of endothelial NOS activity; data not shown). Skeletal muscle contains large amounts of nNOS, the function of which is largely unclear (19). We show here that 7-NI, a potent and selective inhibitor of nNOS activity, reduces skeletal muscle necrosis, suggesting that NO from nNOS contributes to the death of skeletal muscle cells, presumably by causing PARS activation in this tissue. This hypothesis is supported by our findings that AE-ITU, which is a potent inhibitor of both iNOS and nNOS activity, also reduced skeletal muscle necrosis, although there was no induction of iNOS protein in skeletal muscle subjected to ischemia and reperfusion (n = 4; data not shown). In principle, activation of nNOS (e.g., with N-methyl-d-aspartate) results in the generation of amounts of NO that are sufficient to cause PARS activation and neuronal cell death (7). Thus, we propose that an enhanced formation of NO from nNOS contributes to the death of skeletal muscle cells, presumably via PARS activation in this tissue. In contrast, an enhanced formation of oxygen-derived free radicals (but not NO or peroxynitrite) contributes to cell death and PARS activation in cardiomyocytes subjected to ischemia–reperfusion injury.
Figure 4.
Inhibition of NOS activity reduces infarct size in skeletal muscle, but not in the heart. (a) When compared with vehicle-treated controls (open column, n = 9), the NOS inhibitors NG-methyl-l-arginine (3 mg·kg−1 i.a., black column, n = 3), 7-NI (30 mg·kg−1 i.p., gray column, n = 5), and AE-ITU (10 mg·kg−1 i.a., hatched column, n = 3) did not reduce infarct size in the heart. (b) When compared with vehicle-treated controls (open column; n = 18), the NOS inhibitors 7-NI (30 mg·kg−1 i.p., gray column, n = 10) and AE-ITU (10 mg·kg−1 i.v., hatched column, n = 12) caused a significant reduction in the degree of skeletal muscle infarction. ∗, P < 0.05 compared with control (ANOVA followed by Bonferoni’s test).
Summary and Conclusion.
We propose that the DNA damage caused by reactive radicals during the reperfusion of previously ischemic myocardium or skeletal muscle leads to the excessive activation of the DNA repair enzyme PARS, which in turn causes cell death. PARS is essential for DNA repair (1) and, hence, contributes to the efficient maintenance of genome integrity when cells are challenged by genotoxic agents. Thus, a moderate activation of PARS protects genome integrity, while its excessive activation (e.g., during reperfusion of ischemic tissue) may lead to cell death. Although chronic inhibition of the activity of PARS may well lead to side effects, we propose that a transient inhibition of PARS activity is a novel approach for the therapy of ischemia–reperfusion injury of the heart or skeletal muscle (and potentially other organs/tissues). When compared with other drugs that reduce infarct size, PARS inhibitors may offer the advantage that they are able to salvage previously ischemic tissue, even when administered with the onset of reperfusion, without causing any hemodynamic effects.
Acknowledgments
C.T. is a Senior Research Fellow of the British Heart Foundation (FS 96/018). J.B. is supported by a Ph.D. Studentship of the British Heart Foundation (FS 96/015). F.P.M. is a Clinical Lecturer in Surgery at University College London.
Footnotes
Abbreviations: PARS, poly(ADP ribose) synthetase; LAL, first antero-lateral branch of the left coronary artery; NOS, NO synthase; nNOS, neuronal NOS; iNOS, inducible NOS; 7-NI, 7-nitroindazole; 3-AB, 3-aminobenzamide; AE-ITU, aminoethyl-isothiourea.
References
- 1.Satoh M S, Lindahl T. Nature (London) 1992;356:356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 2.Ikai K, Ueda K. J Histochem Cytochem. 1983;31:1261–1264. doi: 10.1177/31.11.6311893. [DOI] [PubMed] [Google Scholar]
- 3.Schraufstatter I U, Hyslop P A, Hinshaw D B, Spragg R G, Sklar L A, Cochrane C G. Proc Natl Acad Sci USA. 1986;83:4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyslop P A, Hinshaw D B, Halsey W A, Schraufstatter I U, Sauerheber R D, Spraggs R G, Jackson J H, Cochrane C G. J Biol Chem. 1988;263:1665–1675. [PubMed] [Google Scholar]
- 5.Thies R L, Autor A P. Arch Biochem Biophys. 1991;286:353–363. doi: 10.1016/0003-9861(91)90051-j. [DOI] [PubMed] [Google Scholar]
- 6.Heller B, Wang Z Q, Wagner E F, Radons J, Burkle A, Fehsel F, Burkhart V, Kolb H. J Biol Chem. 1995;270:11176–11180. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Dawson V L, Dawson T M, Snyder S H. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 8.Szabó C, Zingarelli B, O’Conner M, Salzman A L. Proc Natl Acad Sci USA. 1996;93:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aalto T K, Raivio K O. Pediatr Res. 1993;34:572–576. doi: 10.1203/00006450-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kloner R A, Przyklenk K, Whittaker P. Circulation. 1989;80:1115–1127. doi: 10.1161/01.cir.80.5.1115. [DOI] [PubMed] [Google Scholar]
- 11.Kukreja R C, Hess M L. Cardiovasc Res. 1992;26:641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- 12.Thiemermann C, Thomas R G, Vane J R. Br J Pharmacol. 1988;97:401–408. doi: 10.1111/j.1476-5381.1989.tb11967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Kimpe S J, Hunter M L, Bryant C E, Thiemermann C, Vane J R. Br J Pharmacol. 1995;114:1317–1323. doi: 10.1111/j.1476-5381.1995.tb13349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant C E, Tomlinson A, Mitchell J A, Thiemermann C, Willoughby D. J Endocrinol. 1995;146:149–157. doi: 10.1677/joe.0.1460149. [DOI] [PubMed] [Google Scholar]
- 15.Banasik M, Komura H, Shimoyama M, Ueda K. J Biol Chem. 1992;267:1569–1575. [PubMed] [Google Scholar]
- 16.Rankin P W, Jacobson E L, Benjamin R C, Moss J, Jacobson M K. J Biol Chem. 1989;264:4312–4317. [PubMed] [Google Scholar]
- 17.Southan G J, Szabó C, Thiemermann C. Br J Pharmacol. 1995;114:510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bland-Ward P A, Moore P K. Life Sci. 1995;57:131–135. doi: 10.1016/0024-3205(95)02046-l. [DOI] [PubMed] [Google Scholar]
- 19.Kobzik L, Reid M B, Bredt D S, Stamler J S. Nature (London) 1994;372:504–505. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]