Abstract
The Bcl-2 protein family is characterized by the ability to modulate cell death, and members of this family share two highly conserved domains called Bcl-2 homology 1 (BH1) and 2 (BH2) which have been shown to be critical for the death-repressor activity of Bcl-2 and Bcl-xL. Through sequence analysis we identified a novel viral Bcl-2 homolog, designated KSbcl-2, from human herpesvirus 8 (HHV8) or Kaposi sarcoma-associated herpesvirus. The overall amino acid sequence identity between KSbcl-2 and other Bcl-2 homologs is low (15–20%) but concentrated within the BH1 and BH2 regions. Overexpression of KSbcl-2 blocked apoptosis as efficiently as Bcl-2, Bcl-xL, or another viral Bcl-2 homolog encoded by Epstein–Barr virus, BHRF1. Interestingly, KSbcl-2 neither homodimerizes nor heterodimerizes with other Bcl-2 family members, suggesting that KSbcl-2 may have evolved to escape any negative regulatory effects of the cellular Bax and Bak proteins. Furthermore, the herpesvirus Bcl-2 homologs including KSbcl-2, BHRF1, and ORF16 of herpesvirus saimiri contain poorly conserved Bcl-2 homology 3 (BH3) domains compared with other mammalian Bcl-2 homologs, implying that BH3 may not be essential for anti-apoptotic function. This is consistent with our observation that amino acid substitutions within the BH3 domain of Bcl-xL had no effect on its death-suppressor activity.
DNA sequences of the eighth human herpesvirus were recognized in Kaposi sarcoma (KS) tissues of AIDS patients through the application of representational difference analysis to discern DNA sequences in KS that were absent from normal DNA (1). This virus, known as human herpesvirus 8 (HHV8) or KS-associated herpesvirus, is more closely related to the γ-2 herpesvirus, herpesvirus saimiri (HVS) than to Epstein–Barr virus (EBV) (a γ-1 herpesvirus) based on DNA sequence analysis (1, 2). The preferential detection of HHV8 in KS lesions together with epidemiologic evidence implicate HHV8 in the etiology of KS (1). Recent serologic data suggest that HHV8 infections are acquired in the months to years prior to the onset of KS (3–5). However, HHV8 may be endemic in some geographic locations (5). Several cell lines derived from body cavity-based lymphomas (BCBL) are latently infected with HHV8 and productive lytic infection can be triggered in some of these lines upon addition of phorbol ester or butyrate (4, 6), which is similar to the lymphomagenic viruses EBV and HVS. The role of HHV8 in the etiology of KS or BCBL is not known. bcl-2 was first identified at the t(14;18) chromosomal breakpoint of follicular B cell lymphomas (7, 8). It is a potent cell death-suppressor (9) and represents an unique type of protooncogene that extends cell survival by inhibiting apoptosis rather than promoting cell proliferation (10). Both EBV and HVS encode bcl-2 homologs called BHRF1 and ORF16, respectively (7, 11). Gene transfer studies have shown that BHRF1 can block apoptosis induced by various stimuli such as withdrawal of serum (12) or growth factors (13), treatment with anticancer agents, and infection with heterologous virus (14). Overexpression of ORF16 from HVS also blocks Sindbis virus-induced apoptosis (V.E. Nava and J.M.H., unpublished data). Based on the similarity between HHV8, HVS, and EBV, we sought to identify a bcl-2 homolog encoded by HHV8 and to test if this homolog can block apoptosis.
MATERIALS AND METHODS
Cloning of KSbcl-2.
Total DNA was prepared from a BCBL obtained from an AIDS patient, partially digested with Sau3A, and fractionated by sucrose density gradient centrifugation. The fraction containing 19- to 20-kb DNA was ligated into the BamHI site of EMBL3 (Stratagene). Partial mapping of the 19-kb λ clone 7.1 indicated its colinearity with the region of HVS containing ORF16.
Plasmid Constructions.
The KSbcl-2 open reading frame (ORF) was amplified by PCR from λ clone 7.1 with primers containing BglII restriction sites (5′-AGTCAGATCTACCATGGACGAGGACGTTTTG, 5′-GCCAAGATCTTTATCTCCTGCTCATCGC) and inserted into a modified pSG5 (Stratagene) vector resulting in the addition of an N-terminal hemagglutinin (HA) epitope tag. The sequence of HA-KSbcl-2 was confirmed by the dideoxy sequencing method. An EcoRI–XbaI fragment containing HA-KSbcl-2 was blunt end ligated into the BstEII site of the double subgenomic Sindbis virus vector (dsSV) in both orientations (15, 16). BglII fragments of human bcl-2, human bcl-xL, HA-tagged human bax, and HA-tagged human bak were each cloned into the BglII site of pSG5 for in vitro translation. The triple amino acid substitutions (residues 92, 95, and 96) within the Bcl-2 homology 3 (BH3) domain of Bcl-xL were accomplished by oligonucleotide-directed mutagenesis via two-step PCR. Mutated bcl-xL was inserted into the dsSV vector and mutations were confirmed by DNA sequencing.
Production of Recombinant Sindbis Virus.
dsSV vectors containing KSbcl-2 in both orientations, chloramphenicol acetyltransferase (CAT), bcl-2, bcl-xL, BHRF1, or the BH3 mutant of bcl-xL were each linearized with XhoI and in vitro transcribed using SP6 RNA polymerase. Stocks of recombinant viruses were generated by transfecting the infectious RNA into BHK (baby hamster kidney) cells and collecting the supernatant at 24 h posttransfection. Virus titers were determined by standard plaque assays.
Cell Viability and Immunoblotting.
BHK cells were infected with different recombinant dsSV viruses at a multiplicity of infection (moi) of 5 plaque forming units per cell, and cell viability was determined by the trypan blue exclusion method at 48 h postinfection. At 16 h postinfection cell lysates were prepared from BHK cells infected with recombinant viruses at a moi of 5 and were immunoblotted with 12CA5 anti-HA antibody (Berkeley Antibody, Richmond, CA) and detected by ECL (Amersham).
Coimmunoprecipitation.
Different combinations of Bcl-2 family members were cotranslated in vitro in the presence of [35S]methionine using reticulocyte lysates (Promega) and analyzed by 12% SDS/PAGE and autoradiography. Lysates (5–10 μl) were immunoprecipitated with 12CA5 antibody and analyzed by 12% SDS/PAGE and autoradiography.
Detection of KSbcl-2 in BCBL-1 Cells.
Using a Quickprep Micro mRNA Purification Kit (Pharmacia), poly(A)+ RNA was prepared from BCBL-1 cells (6) that were untreated or treated with 20 ng/ml 12-O-tetradecanoylphorbol 13-acetate (TPA; Sigma) for 48 h. Complementary DNA was transcribed using a cDNA Synthesis Kit (Boehringer Mannheim) and PCR amplified using primers specific for KSbcl-2 (described above) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5′ACCACCATGGAGAAGGCTGG, 5′CTCAGTGTAGCCCAGGATGC). Controls included DNase treatment of RNA and omission of reverse transcriptase. PCR products were separated on a 0.8% agarose gel and detected with ethidium bromide.
RESULTS
HHV8 Encodes a bcl-2 Homolog.
A 6.6-kb BamHI subfragment of HHV8 genomic DNA from λ clone 7–1 was predicted to contain a bcl-2 homolog by analogy with the genome organization of HVS through mapping studies. This subfragment was fully sequenced and an ORF encoding a bcl-2 homolog, designated as KSbcl-2, was identified in the HHV8 genome at an equivalent position and orientation to that of ORF16 in HVS. In both genomes, the bcl-2 homologs are located between blocks of homologous genes that are conserved among all of the γ herpesviruses. However, the bcl-2 homolog in EBV is located between different conserved gene blocks compared with the homologs present in HVS and HHV8. Therefore, the acquisitions of bcl-2 homologs by γ 1 (EBV) versus γ 2 (HVS and HHV8) viruses may have been independent evolutionary events, although other explanations are possible. Alignment of the amino acid sequence of KSbcl-2 with that of several Bcl-2 family members (Bcl-2, Bcl-xL, Bax, Bak, ORF16, and BHRF1) indicate that KSbcl-2 shares colinear homology while significant amino acid identity is limited to the conserved BH1 and BH2 domains (Fig. 1A). The overall amino acid sequence identity between KSbcl-2 and other Bcl-2 family members is low (15–20%) but within the range of identities observed for most family members (Fig. 1B). Interestingly, the herpesvirus Bcl-2 proteins contain poorly conserved BH3 domains compared with other mammalian Bcl-2 homologs. Furthermore, the unstructured loop (amino acids 30–80) found in Bcl-xL and presumably Bcl-2 (17) is lacking in the viral homologs, potentially representing loss of regulatory mechanisms imposed on the cellular but not viral homologs. The viral homologs all contain a stretch of hydrophobic residues at their carboxy termini, ending with basic residues. By analogy to Bcl-2 and BHRF1, these hydrophobic regions are expected to mediate intracellular membrane localization (18–20).
Figure 1.
Alignment of KSbcl-2 amino acid sequence with other viral and human Bcl-2 family members. (A) Shaded areas indicate amino acid identity in four or more entries, defining the consensus sequence. Brackets indicate the homology domains BH1-4, and the C-terminal hydrophobic domain is underlined. (B) The percent amino acid identity between KSbcl-2 and other family members was calculated using geneworks. The GenBank accession number for KSbcl-2 is U67773U67773.
Overexpression of KSbcl-2 Inhibits Sindbis Virus-Induced Apoptosis.
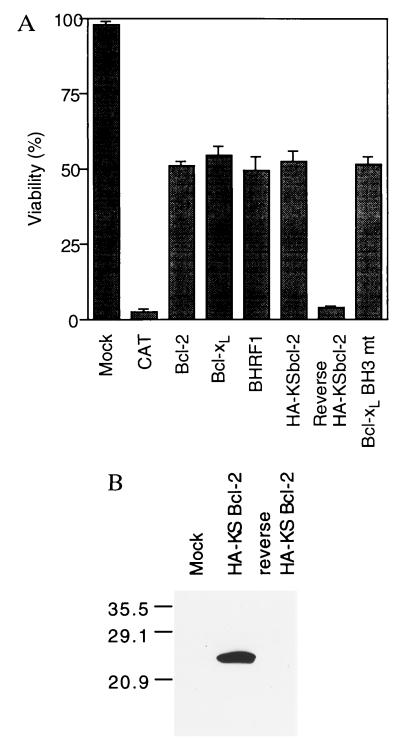
Sindbis virus, an alphavirus, has been shown to induce typical apoptosis of infected cells (21–23). We have established a Sindbis virus vector system to test the ability of candidate death-regulatory genes to delay or accelerate Sindbis virus-induced apoptosis (15, 16). The putative KSbcl-2 coding sequence with a N-terminal HA-tag was expressed via the Sindbis virus vector and proved to be equivalent to Bcl-2, Bcl-xL, and BHRF1 in its ability to inhibit Sindbis virus-induced apoptosis as indicated by ≈50% cell viabilities at 48 h postinfection compared to 2–3% with an irrelevant gene (CAT) or with KSbcl-2 in the reverse orientation (Fig. 2A). We and others have found that the HA-tag has no effect on the anti-apoptotic activity of Bcl-2 homologs (16, 24). To verify the expression of KSbcl-2 protein, infected cell lysates were analyzed by immunoblotting with anti-HA antibodies. A protein of 24 kDa was observed only in cells infected with the recombinant virus encoding HA-KSbcl-2 (Fig. 2B).
Figure 2.
KSbcl-2 inhibits cell death. (A) BHK cells were infected at a moi of 5 with recombinant virus encoding HA-tagged KSbcl-2 in the forward or reverse orientation. Alternatively, cells were infected with recombinant viruses encoding wild-type human Bcl-xL, human Bcl-xL with mutations in the BH3 region, human Bcl-2, BHRF1, or CAT. Cell viability was determined at 48 h postinfection (or mock infection) by trypan blue exclusion in three or more independent experiments. Immunofluorescence assays indicated that all of the cells were infected (data not shown), and a variety of techniques have demonstrated that virtually all cells infected with Sindbis virus die by apoptosis (21–23). (B) Lysates prepared from BHK cells infected with recombinant viruses encoding KSbcl-2 or reverse KSbcl-2 were immunoblotted with 12CA5 anti-HA antibody. The positions of molecular weight standards are indicated.
The poor conservation of BH3 domains in the viral homologs and the apparent absence of BH3 in ORF16 suggests that BH3 is not essential for anti-death activity. To assess the role of the BH3 domain in the death-suppressor activity of Bcl-xL, amino acid substitutions were introduced into the BH3 domain of Bcl-xL (Glu to Gln change at residue 92, Asp to Ala change at residue 95, and Glu to Asp change at residue 96) and the anti-death function was assayed using the Sindbis virus vector system. The BH3 mutant was as efficient as wild-type Bcl-xL at inhibiting cell death (Fig. 2A), indicating that BH3 may not be directly involved in the death-suppressor activity of Bcl-xL.
KSbcl-2 Neither Homodimerizes Nor Heterodimerizes with Other Bcl-2 Homologs.
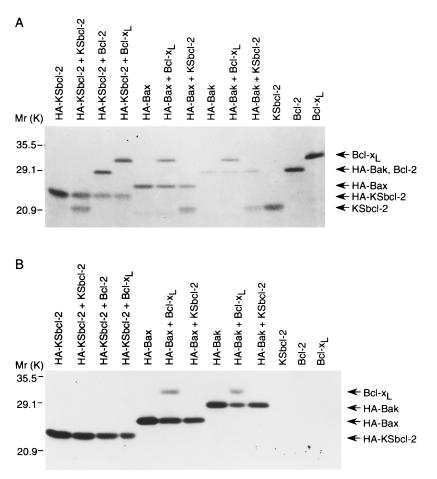
One common feature among the Bcl-2 family members is that they often homodimerize or heterodimerize with other family members (25, 26). Although neither homodimerization nor heterodimerization is required for the death-repressor activity of Bcl-xL, its heterodimerization with Bax or Bak negatively regulates the anti-apoptotic activity of Bcl-xL (16). Likewise, Bax and Bak appear to have a pro-death function other than sequestering Bcl-2 and Bcl-xL (27), but may still competitively inhibit the binding of a downstream target protein to Bcl-xL. To assess the dimerization of KSbcl-2, we performed coimmunoprecipitation assays of 35S-labeled in vitro cotranslated proteins using anti-HA antibodies. KSbcl-2, Bcl-2, and Bcl-xL failed to coprecipitate with HA-KSbcl-2 (Fig. 3B, lanes 2–4). KSbcl-2 also failed to coprecipitate with HA-Bax and HA-Bak (Fig. 3B, lanes 7 and 10). However, Bcl-xL was coprecipitated with both HA-Bax and HA-Bak as expected (Fig. 3B, lanes 6 and 9). To demonstrate that similar amounts of proteins were present in the translation mix, cotranslated proteins were analyzed before precipitation (Fig. 3A). The lack of interaction between KSbcl-2 and other Bcl-2 homologs was further confirmed by yeast two-hybrid interaction assays (data not shown). Thus, KSbcl-2 neither homodimerizes nor heterodimerizes with Bcl-2, Bcl-xL, Bax, or Bak.
Figure 3.
Lack of heterodimerization between KSbcl-2 and other Bcl-2 family members. (A) The indicated proteins were co-translated in vitro in the presence of [35S]methionine, separated on a 12% SDS gel, and analyzed by autoradiography. (B) Cotranslated proteins were immunoprecipitated with 12CA5 anti-HA antibody and precipitates were analyzed by SDS/PAGE and autoradiography. The positions of molecular weight markers are indicated. Note that HA-tagged Bak comigrates with Bcl-2.
KSbcl-2 Is Expressed During the Lytic Viral Replication Cycle.
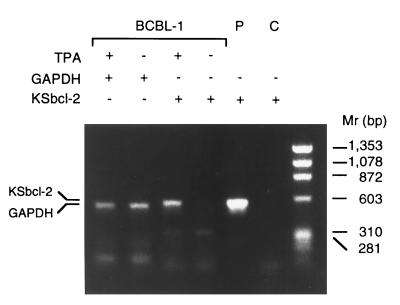
Renne et al. (6) reported that a BCBL cell line, designated BCBL-1, is infected with HHV8 and lacks EBV sequences. Furthermore, treatment of BCBL-1 cells with a phorbol ester (TPA) significantly induces HHV8 gene expression. By analogy with EBV, which can be reactivated by treatment with TPA, TPA treatment of BCBL-1 cells apparently induces latent HHV8 to enter the lytic replication cycle, resulting in the production of mature virions (6). To determine if KSbcl-2 was expressed in the latent or lytic phases of the viral replication cycle, BCBL-1 cells were analyzed by reverse transcription–PCR for expression of KSbcl-2 (Fig. 4). Although roughly equivalent amounts of cellular GAPDH were detected in TPA-treated and untreated cells, KSbcl-2 was detected only in TPA-treated samples, indicating that KSbcl-2 is a lytic cycle gene, consistent with the fact that BHRF1 of EBV is abundantly expressed early in the lytic replication cycle (18, 19).
Figure 4.
KSbcl-2 is expressed during the lytic viral replication cycle. Cellular GAPDH or HHV8 KSbcl-2 sequences were amplified by reverse transcription–PCR from mRNA isolated from TPA-treated and untreated BCBL-1 cells. PCR products were analyzed on a 0.8% agarose gel and stained with ethidium bromide. The KSbcl-2 PCR product comigrated with a PCR product amplified from plasmid pHYC323 containing the KSbcl-2 ORF (lane P), but was not detected in a control reaction lacking template DNA (lane C).
DISCUSSION
Apoptosis or programmed cell death is an active process of cellular self-destruction with distinctive morphological and biochemical features (28). It is essential for embryonic development and the maintenance of homeostasis of multicellular organisms. Programmed cell death can serve as a defense mechanism against intracellular microbes (29). Virus infections trigger host cell apoptosis, which can either limit virus production or contribute directly to viral pathogenesis (23, 30). Inhibition of apoptosis either by viral genes or by induction of cellular death-suppressors blocks premature death of infected cells and thereby facilitates a persistent infection or prolongs the survival of lyticly infected cells to maximize the production of viral progeny (30, 31). Many viruses encode anti-apoptotic genes, including p35 and iap of baculoviruses (32, 33) and E1b-19K and -55K of adenoviruses (34) that, when deleted, severely impair progeny virus production. Through sequence analysis we have identified a Bcl-2 homolog encoded by HHV8 and have proven its death-suppressor activity using the Sindbis virus vector system. Expression of KSbcl-2 could provide a selective advantage to HHV8 by inhibiting apoptosis either in primary infections or during reactivation from latency, consistent with its expression during the lytic replication cycle.
Prolonged cell survival mediated by the bcl-2 gene predisposes cells to additional genetic changes in conventional oncogenes such as c-myc, thus enhancing tumor progression (35, 36). Within the γ herpesviruses, EBV, HVS, and HHV8 are closely associated with tumors and all encode a bcl-2 homolog. However, BHRF1 is dispensable for in vitro transformation of B-lymphocytes by EBV (37, 38), and therefore may not be directly involved in EBV lymphomagenesis. Nevertheless, BHRF1 blocks epithelial cell differentiation and could potentially contribute to the development of epithelial tumors such as nasopharyngeal carcinoma (39). HHV8 also encodes a cyclin D homolog that can be detected in KS lesions (40). In addition to its role in cell cycle progression and tumorigenesis, cellular cyclin D has also been shown to induce apoptosis (41, 42). Thus, an anti-apoptotic activity could potentially counteract a pro-death signal of cyclin D. Like KSbcl-2, the HHV8 cyclin D homolog is also expressed during the lytic cycle (D.S.B. and J.M.H., unpublished data), raising the possibility that other lytic cycle genes such as KSbcl-2 also may be expressed in KS tumors.
Bcl-2 family members can be divided into two subgroups, death-inhibitory and death-promoting homologs. The former includes Bcl-2 and Bcl-xL (9, 43), while Bax and Bak represent the latter subgroup (24, 44–46). Although it has been debated which subgroup mediates the downstream effects that regulate cell death, site-specific mutagenesis studies of Bcl-xL indicate that Bcl-xL can protect cells from apoptosis by a mechanism independent of Bax and Bak (16). That is, Bcl-xL appears to have a function other than simply sequestering Bax and Bak through heterodimerization. Here, we demonstrated that overexpression of KSbcl-2 blocks Sindbis virus-induced apoptosis at levels comparable to those displayed by Bcl-2, Bcl-xL, and BHRF1 despite its inability to heterodimerize with Bax or Bak. These results further support our hypothesis that the death-inhibitory Bcl-2 homologs have downstream pro-survival functions and are active effectors in blocking apoptosis. Likewise, Bax and Bak may have pro-death activity that is independent of their ability to heterodimerize with the pro-survival family members (47). Mutant Bax lacking the BH3 domain, which is required (and sufficient) for heterodimerization with Bcl-xL, can still antagonize the anti-apoptotic function of Bcl-xL (27, 48). Thus, the pro-survival and pro-death members of the Bcl-2 family may have independent activities. Nevertheless, heterodimerization may serve to regulate the function of Bcl-2 family members through a balance in the ratio of death-suppressors to death-inducers. Because KSbcl-2 fails to bind with Bax or Bak, it could potentially escape negative regulation by Bax or Bak and represent a powerful death-suppressor. Alternatively, unidentified cellular factors may exist that counteract the death-repressor activity of KSbcl-2 and function by a mechanism similar to Bax and Bak. BHRF1 also fails to heterodimerize with Bax. Although BHRF1/Bak heterodimers have been reported, Bak apparently has anti-apoptotic rather than pro-apoptotic activity in B lymphocytes, the target cells for EBV (46, 49). Site-directed mutagenesis of Bcl-2 suggests that homodimerization also is not required for the death-repressor activity of Bcl-2 (50). Furthermore, neither structural (17) nor biochemical analysis (E.H.-Y.C. and J.M.H., unpublished data) suggest that Bcl-xL forms homodimers. The lack of homodimerization of KSbcl-2 provides additional evidence that homodimers may not be the functional moiety for Bcl-2 homologs to block apoptosis.
Although the BH3 domains of Bax and Bak facilitate cell death and mediate heterodimerization with BclxL and Bcl-2, the role of BH3 in Bcl-2 and Bcl-xL is not clear. Interestingly, the Bcl-2 homologs from γ herpesviruses, including BHRF1, ORF16, and KSbcl-2, contain poorly conserved BH3 domains compared with the mammalian homologs but still efficiently protect cells from apoptosis. Furthermore, we demonstrated here that amino acid substitutions within the BH3 domain of Bcl-xL had no effect on its death-repressor activity. This suggests that BH3 is not directly involved in the death-repressor activity of Bcl-2 homologs. Based on the structural analysis of Bcl-xL, BH3 is in close proximity to both BH1 and BH2, which may suggest a regulatory role of BH3 on the death-inhibitory activity of BH1 and BH2 (17). Therefore, the distinct BH3 domains of viral Bcl-2 homologs could potentially represent a virus-specific regulatory mechanism.
Acknowledgments
We thank Shifa Zou for excellent technical assistance. This work was supported by Grants RO1 NS34175 and RO1 CA73581 (J.M.H.) from the National Institutes of Health.
Footnotes
Abbreviations: BCBL, body cavity-based lymphoma; BH1-4, Bcl-2 homology domains 1–4; dsSV, double subgenomic Sindbis virus vector; EBV, Epstein–Barr virus; HHV8, human herpesvirus 8; HVS, herpesvirus saimiri; KS, Kaposi sarcoma; HA, hemagglutinin; CAT, chloramphenicol acetyltransferase; TPA, 12-O-tetradecanoylphorbol 13-acetate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ORF, open reading frame.
Data deposition: The sequence reported in this paper has been deposited in the GenBank data base (accession no. U67773U67773).
References
- 1.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Moore P S, Gao S-J, Dominguez G, Cesarman E, Lungu O, Knowles D M, Garber R, Pellett P E, McGeoch D J, Chang Y. J Virol. 1996;70:549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao S-J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 4.Miller G, Rigsby M O, Heston L, Grogan E, Sun R, Metroka C, Levy J A, Gao S-J, Chang Y, Moore P. N Engl J Med. 1996;334:1292–1297. doi: 10.1056/NEJM199605163342003. [DOI] [PubMed] [Google Scholar]
- 5.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 6.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 7.Cleary M L, Smith S D, Sklar J. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsujimoto Y, Bashir M M, Givol I, Cossman J, Jaffe E, Croce C M. Proc Natl Acad Sci USA. 1987;84:1329–1331. doi: 10.1073/pnas.84.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed J C. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsmeyer S J. Blood. 1992;80:879–886. [PubMed] [Google Scholar]
- 11.Smith C A. Trends Cell Biol. 1995;5:344. doi: 10.1016/s0962-8924(00)89061-3. [DOI] [PubMed] [Google Scholar]
- 12.Henderson S, Hue D, Rowe M, Dawson C, Johnson G, Rickinson A. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayama S, Cazals-Hatem D L, Kitada S, Tanaka S, Miyashita T, Hovey L R, III, Huen D, Rickinson A, Veerapandian P, Krajewski S, Saito K, Reed J. DNA Cell Biol. 1994;13:679–692. doi: 10.1089/dna.1994.13.679. [DOI] [PubMed] [Google Scholar]
- 14.Tarodi B, Subramanian T, Chinnadurai G. Virology. 1994;201:404–407. doi: 10.1006/viro.1994.1309. [DOI] [PubMed] [Google Scholar]
- 15.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng E H -Y, Levine B, Boise L H, Thompson C B, Hardwick J M. Nature (London) 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 17.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S-L, Ng S-C, Fesik S W. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 18.Hardwick J M, Lieberman P M, Hayward S D. J Virol. 1988;62:2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson G R, Luka J, Petti L, Sample J, Birkenbach M, Braun D, Kieff E. Virology. 1987;160:151–161. doi: 10.1016/0042-6822(87)90055-9. [DOI] [PubMed] [Google Scholar]
- 20.Hickish T, Robertson D, Clarke P, Hill M, di Stefano F, Clarke C, Cunningham D. Cancer Res. 1994;54:2808–2811. [PubMed] [Google Scholar]
- 21.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Nature (London) 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 22.Ubol S, Tucker P C, Griffin D E, Hardwick J M. Proc Natl Acad Sci USA. 1994;91:5202–5206. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 25.Sato T, Hanada M, Bodrug S, Irie S, Iwama N, Boise L H, Thompson C B, Golemis E, Fong L, Wang H-G, Reed J C. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sedlak T W, Oltvai Z N, Yang E, Wang K, Boise L H, Thompson C B, Korsmeyer S J. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonian P L, Grillot D A M, Merino R, Nuñez G. J Biol Chem. 1996;271:22764–22772. doi: 10.1074/jbc.271.37.22764. [DOI] [PubMed] [Google Scholar]
- 28.Vaux D L, Haecker G, Strasser A. Cell. 1994;76:777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 29.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 30.Hardwick, J. M. (1997) Adv. Pharmacol., in press. [DOI] [PubMed]
- 31.Shen Y, Shenk T E. Curr Biol. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 32.Clem R J, Fechheimer M, Miller L K. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 33.Crook N E, Clem R J, Miller L K. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White E. Virology. 1994;5:341–348. [Google Scholar]
- 35.Strasser A, Harris A W, Bath M L, Cory S. Nature (London) 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 36.McDonnell T J, Korsmeyer S J. Nature (London) 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 37.Marchini A, Tomkinson B, Cohen J I, Kieff E. J Virol. 1991;65:5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee M-A, Yates J L. J Virol. 1992;66:1899–1906. doi: 10.1128/jvi.66.4.1899-1906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson C W, Eliopoulos A G, Dawson J, Young L S. Oncogene. 1995;9:69–77. [PubMed] [Google Scholar]
- 40.Chang Y, Moore P S, Talbot S J, Boshoff C H, Zarkowska T, Godden-Kent D, Paterson H, Weiss R A, Mittnacht S. Nature (London) 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 41.Motokura T, Arnold A. Curr Opin Genet Dev. 1993;3:5–10. doi: 10.1016/s0959-437x(05)80334-x. [DOI] [PubMed] [Google Scholar]
- 42.Kranenburg O, van der EB A J, Zantema A. EMBO J. 1996;15:46–54. [PMC free article] [PubMed] [Google Scholar]
- 43.Boise L H, González-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nuñez G, Thompson C B. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 44.Farrow S N, White J H M, Martinou I, Raven T, Pun K-T, Grinham C J, Martinou J-C, Brown R. Nature (London) 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 45.Chittenden T, Harrington E A, O’Connor R, Flemington C, Lutz R J, Evan G I, Guild B C. Nature (London) 1995;374:733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 46.Kiefer M C, Brauer M J, Powers V C, Wu J J, Umansky S R, Tomei L D, Barr P J. Nature (London) 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 47.Knudson C M, Tung K S K, Tourtellotte W G, Brown G A J, Korsmeyer S J. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 48.Chittenden T, Flemington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theodorakis P, D’Sa-Eipper C, Subramanian T, Chinnadurai G. Oncogene. 1996;12:1707–1713. [PubMed] [Google Scholar]
- 50.Yin X-M, Oltvai Z N, Korsmeyer S J. Nature (London) 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]