Abstract
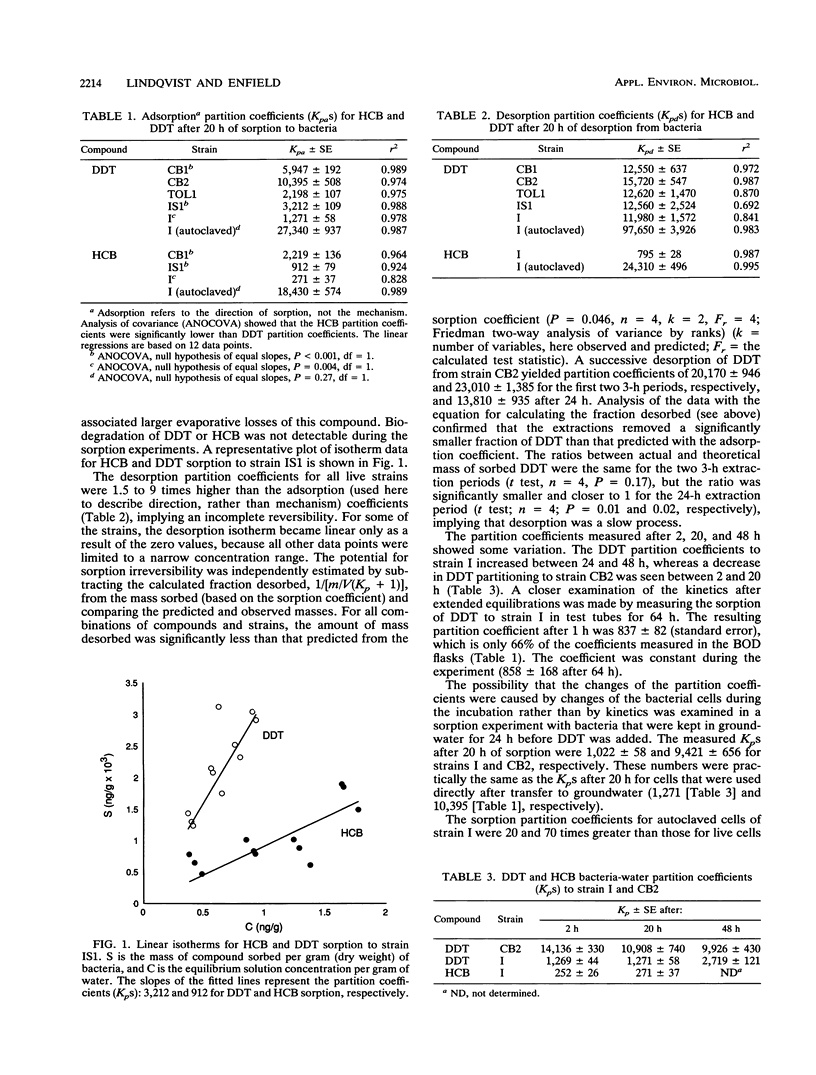
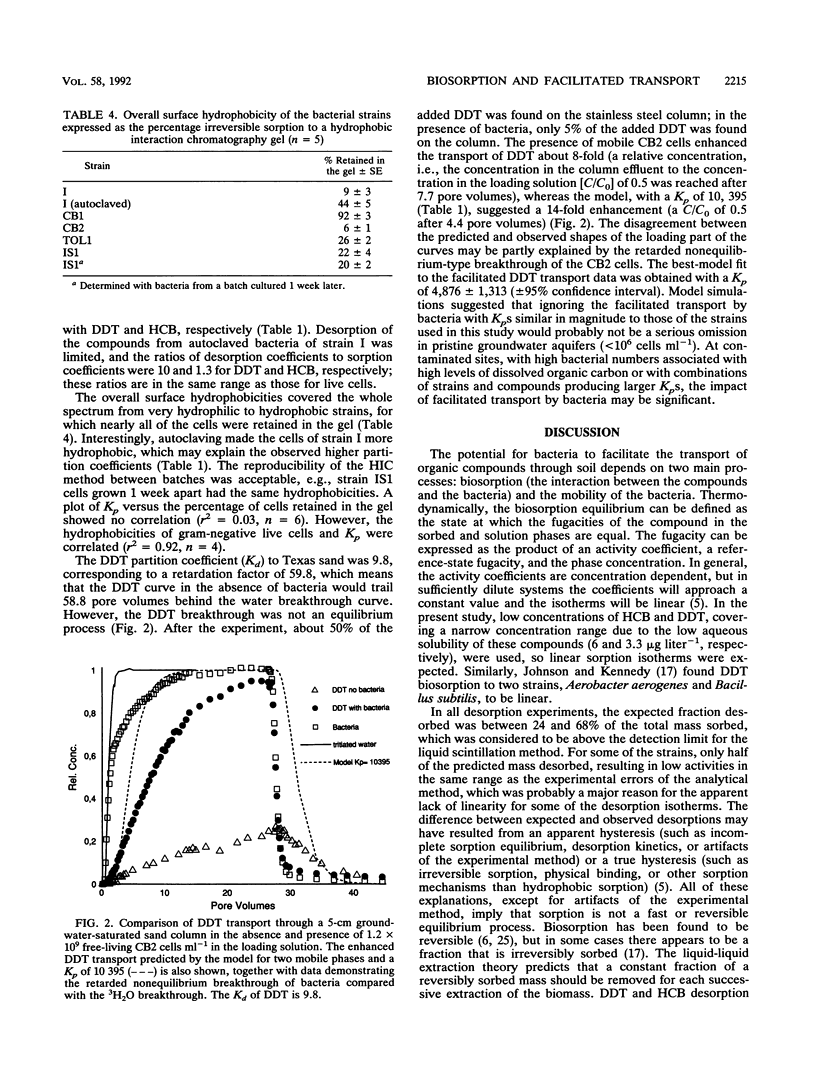
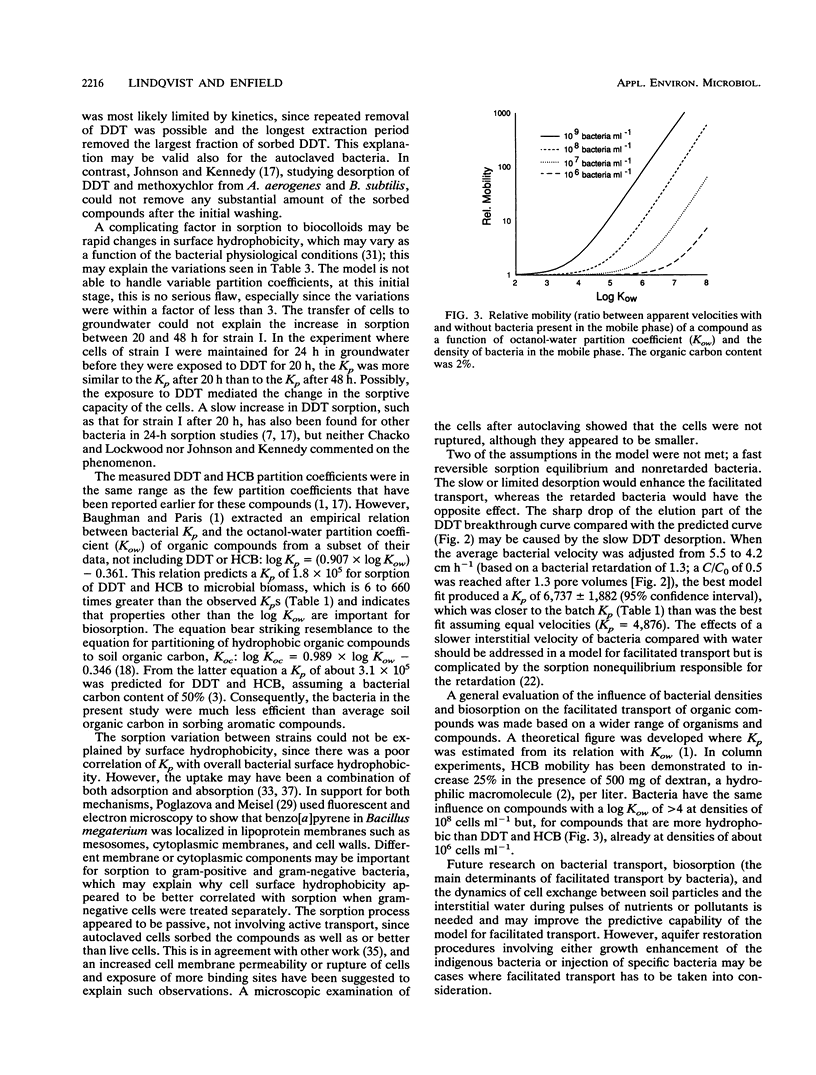
The potential for enhanced mobility of hydrophobic pollutants by cotransport with bacteria in saturated soils was evaluated from measurements of biosorption of 14C-labeled hexachlorobenzene and dichlorodiphenyltrichloroethane (DDT) to five strains of soil and sewage bacteria. The sorption process could be described by a linear partition equation and appeared to be reversible, but desorption kinetics were slow and/or partly irreversible. The DDT partition coefficients varied with equilibration time, possibly reflecting DDT-induced changes in the physiology of the bacteria. The partition coefficients, normalized to the masses of the bacteria, ranged from 250 to 14,000 for live cells, but the largest coefficients were associated with autoclaved cells of a Pseudomonas sp. The sorptive capacity of the bacterial biomass was greater for DDT than for hexachlorobenzene but was not correlated to overall bacterial hydrophobicity, measured by hydrophobic interaction chromatography. In a column study, 1.2 x 10(9) cells of a Bacillus sp. strain per ml enhanced DDT transport about 8-fold, whereas an advective-dispersive-sorptive equilibrium model for two mobile phases, water and free-living bacteria, suggested a 14-fold enhancement, based on the DDT partition coefficient. The disagreement was in part due to a retarded nonequilibrium movement of the bacteria. Model calculations based on literature data covering a wide range of organisms and compounds suggested that 10(6) cells ml-1 would increase the mobility of very hydrophobic compounds (log octanol-water partition coefficient [K(ow) of greater than or equal to 6), whereas higher densities of bacteria (10(8) cells ml-1) would have a significant impact on compounds with a log K(ow) of greater than or equal to 4.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughman G. L., Paris D. F. Microbial bioconcentration of organic pollutants from aquatic systems -- a critical review. Crit Rev Microbiol. 1981;8(3):205–228. doi: 10.3109/10408418109085079. [DOI] [PubMed] [Google Scholar]
- Bratbak G., Dundas I. Bacterial dry matter content and biomass estimations. Appl Environ Microbiol. 1984 Oct;48(4):755–757. doi: 10.1128/aem.48.4.755-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko C. I., Lockwood J. L. Accumulation of DDT and dieldrin by microorganisms. Can J Microbiol. 1967 Aug;13(8):1123–1126. doi: 10.1139/m67-153. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B., Hermansson M., Kjelleberg S., Norkrans B. The hydrophobicity of bacteria - an important factor in their initial adhesion at the air-water interface. Arch Microbiol. 1981 Jan;128(3):267–270. doi: 10.1007/BF00422527. [DOI] [PubMed] [Google Scholar]
- Gannon J. T., Manilal V. B., Alexander M. Relationship between Cell Surface Properties and Transport of Bacteria through Soil. Appl Environ Microbiol. 1991 Jan;57(1):190–193. doi: 10.1128/aem.57.1.190-193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C., Wilson J. T. Microbial ecology of the terrestrial subsurface. Adv Appl Microbiol. 1988;33:107–172. doi: 10.1016/s0065-2164(08)70206-5. [DOI] [PubMed] [Google Scholar]
- Johnson B. T., Kennedy J. O. Biomagnification of p, p'-DDT and methoxychlor by bacteria. Appl Microbiol. 1973 Jul;26(1):66–71. doi: 10.1128/am.26.1.66-71.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. S., Finnerty W. R. Microbial assimilation of hydrocarbons. I. The fine-structure of a hydrocarbon oxidizing Acinetobacter sp. Arch Microbiol. 1975;102(2):75–83. doi: 10.1007/BF00428349. [DOI] [PubMed] [Google Scholar]
- Lal R., Saxena D. M. Accumulation, metabolism, and effects of organochlorine insecticides on microorganisms. Microbiol Rev. 1982 Mar;46(1):95–127. doi: 10.1128/mr.46.1.95-127.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogram A. V., Jessup R. E., Ou L. T., Rao P. S. Effects of sorption on biological degradation rates of (2,4-dichlorophenoxy) acetic acid in soils. Appl Environ Microbiol. 1985 Mar;49(3):582–587. doi: 10.1128/aem.49.3.582-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Lewis D. L. Accumulation of methoxychlor by microorganisms isolated from aqueous systems. Bull Environ Contam Toxicol. 1976 Jan;15(1):24–32. doi: 10.1007/BF01686192. [DOI] [PubMed] [Google Scholar]
- Paris D. F., Lewis D. L., Barnett J. T. Bioconcentration of toxaphene by microorganisms. Bull Environ Contam Toxicol. 1977 May;17(5):564–572. doi: 10.1007/BF01685979. [DOI] [PubMed] [Google Scholar]
- Poglazova M. N., Meisel' M. N. Lokalizatsiia benz(a)pirena v bakterial'nykh kletkskh. Mikrobiologiia. 1971 Nov-Dec;40(6):1050–1053. [PubMed] [Google Scholar]
- Wedemeyer G. Uptake of 2,4-dichlorophenoxyacetic acid by Pseudomonas fluorescens. Appl Microbiol. 1966 Jul;14(4):486–491. doi: 10.1128/am.14.4.486-491.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



