Abstract
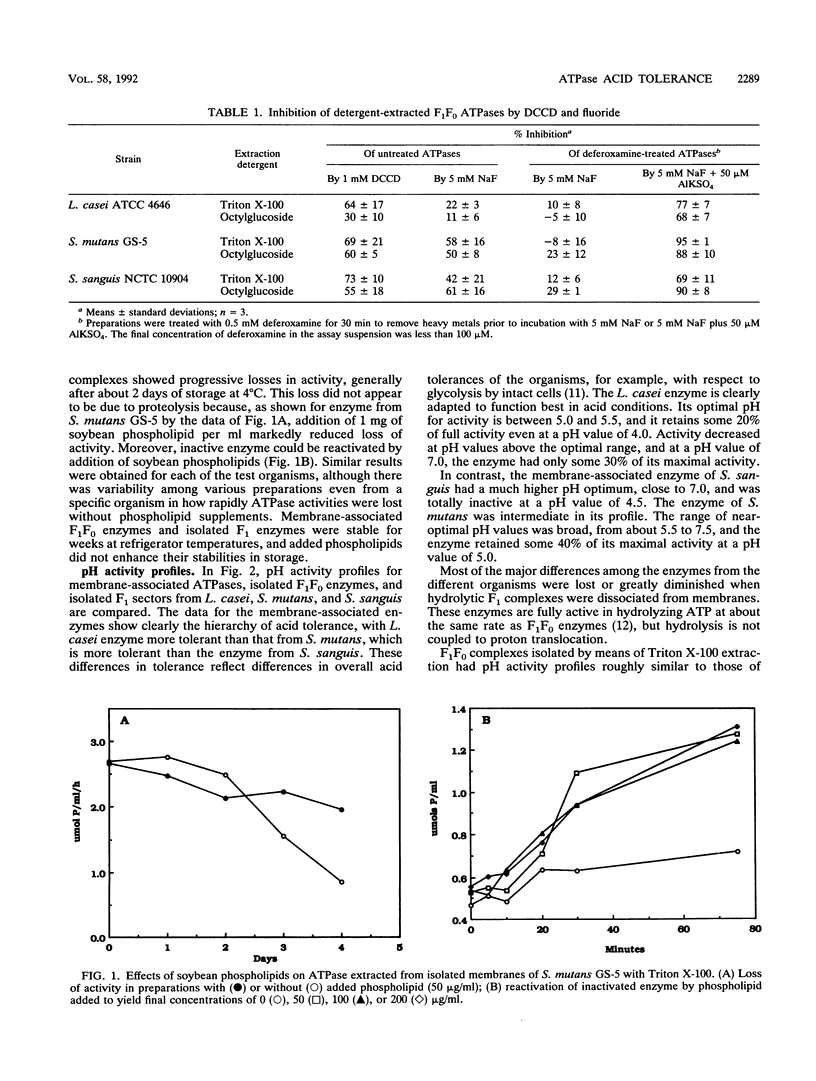
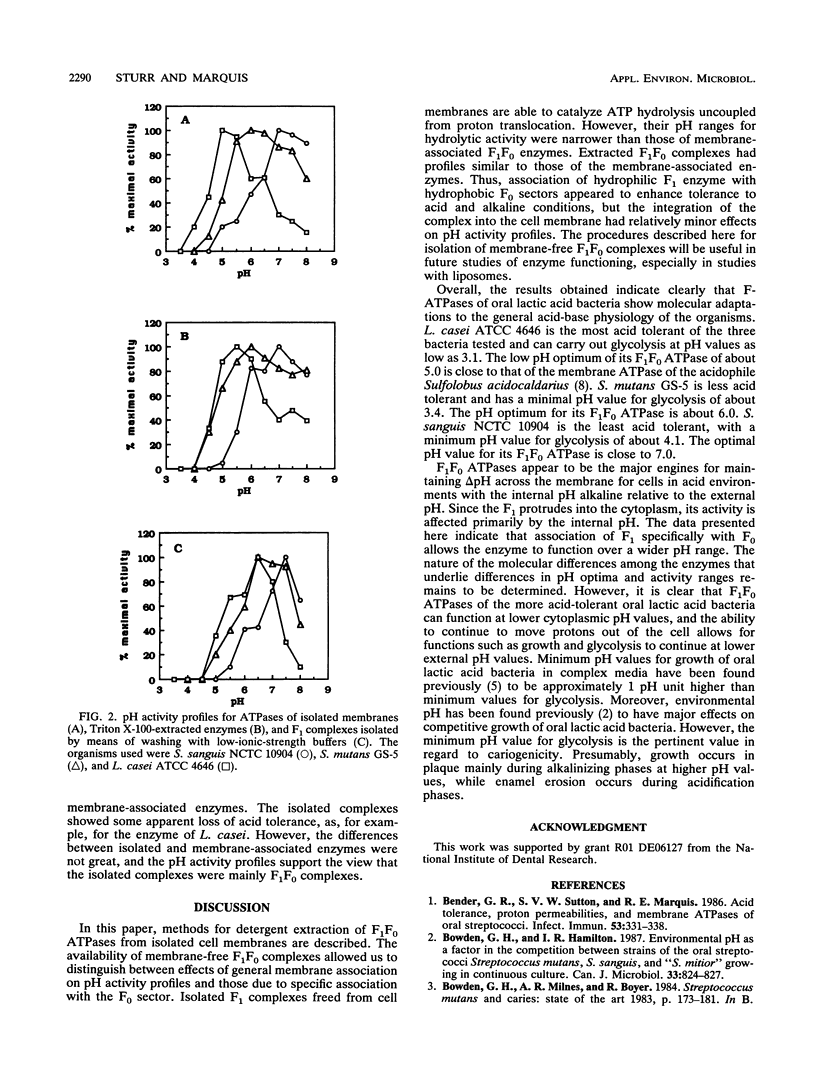
pH activity profiles and inhibitor sensitivities were compared for membrane ATPases isolated from three oral lactic acid bacteria, Lactobacillus casei ATCC 4646, Streptococcus mutans GS-5, and Streptococcus sanguis NCTC 10904, with, respectively, high, moderate, and low levels of acid tolerance. Membranes containing F1F0 ATPases were isolated by means of salt lysis of cells treated with muralytic enzymes. Membrane-free F1F0 complexes were then isolated from membranes by detergent extraction with Triton X-100 or octylglucoside. Finally, F1 complexes free of the proton-conducting F0 sector were obtained by washing membranes with buffers of low ionic strength. The pH activity profiles of the membrane-associated enzymes reflected the general acid tolerances of the organisms from which they were isolated; for example, pH optima were approximately 5.5, 6.0, and 7.0, respectively, for enzymes from L. casei, S. mutans, and S. sanguis. Roughly similar profiles were found for membrane-free F1F0 complexes, which were stabilized by phospholipids against loss of activity during storage. However, profiles for F1 enzymes were distinctly narrower, indicating that association with F0 and possibly other membrane components enhanced tolerance to both acid and alkaline media. All of the enzymes were found to have similar sensitivities to Al-F complexes, but only F1F0 enzymes were highly sensitive to dicyclohexylcarbodiimide. The procedures described for isolation of membrane-free F1F0 forms of the enzymes from oral lactic acid bacteria will be of use in future studies of the characteristics of the enzymes, especially in studies with liposomes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender G. R., Sutton S. V., Marquis R. E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986 Aug;53(2):331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden G. H., Hamilton I. R. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and "S. mitior" growing in continuous culture. Can J Microbiol. 1987 Sep;33(9):824–827. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- Casiano-Colón A., Marquis R. E. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol. 1988 Jun;54(6):1318–1324. doi: 10.1128/aem.54.6.1318-1324.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi J., Wakagi T., Oshima T., Yoshida M. Purification and properties of the ATPase solubilized from membranes of an acidothermophilic archaebacterium, Sulfolobus acidocaldarius. J Biochem. 1987 Dec;102(6):1379–1387. doi: 10.1093/oxfordjournals.jbchem.a122184. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E. Diminished acid tolerance of plaque bacteria caused by fluoride. J Dent Res. 1990 Feb;69(Spec No):672–683. doi: 10.1177/00220345900690S130. [DOI] [PubMed] [Google Scholar]
- Senior A. E. ATP synthesis by oxidative phosphorylation. Physiol Rev. 1988 Jan;68(1):177–231. doi: 10.1152/physrev.1988.68.1.177. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Downie J. A., Cox G. B., Gibson F., Langman L., Fayle D. R. The uncA gene codes for the alpha-subunit of the adenosine triphosphatase of Escherichia coli. Electrophoretic analysis of uncA mutant strains. Biochem J. 1979 Apr 15;180(1):103–109. doi: 10.1042/bj1800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solioz M., Fürst P. Purification of the ATPase of Streptococcus faecalis. Methods Enzymol. 1988;157:680–689. doi: 10.1016/0076-6879(88)57115-x. [DOI] [PubMed] [Google Scholar]
- Sturr M. G., Marquis R. E. Inhibition of proton-translocating ATPases of Streptococcus mutans and Lactobacillus casei by fluoride and aluminum. Arch Microbiol. 1990;155(1):22–27. doi: 10.1007/BF00291269. [DOI] [PubMed] [Google Scholar]
- Sutton S. V., Marquis R. E. Membrane-associated and solubilized ATPases of Streptococcus mutans and Streptococcus sanguis. J Dent Res. 1987 Jun;66(6):1095–1098. doi: 10.1177/00220345870660060201. [DOI] [PubMed] [Google Scholar]
- ten Cate J. M., Duijsters P. P. Influence of fluoride in solution on tooth demineralization. I. Chemical data. Caries Res. 1983;17(3):193–199. doi: 10.1159/000260667. [DOI] [PubMed] [Google Scholar]


