Abstract
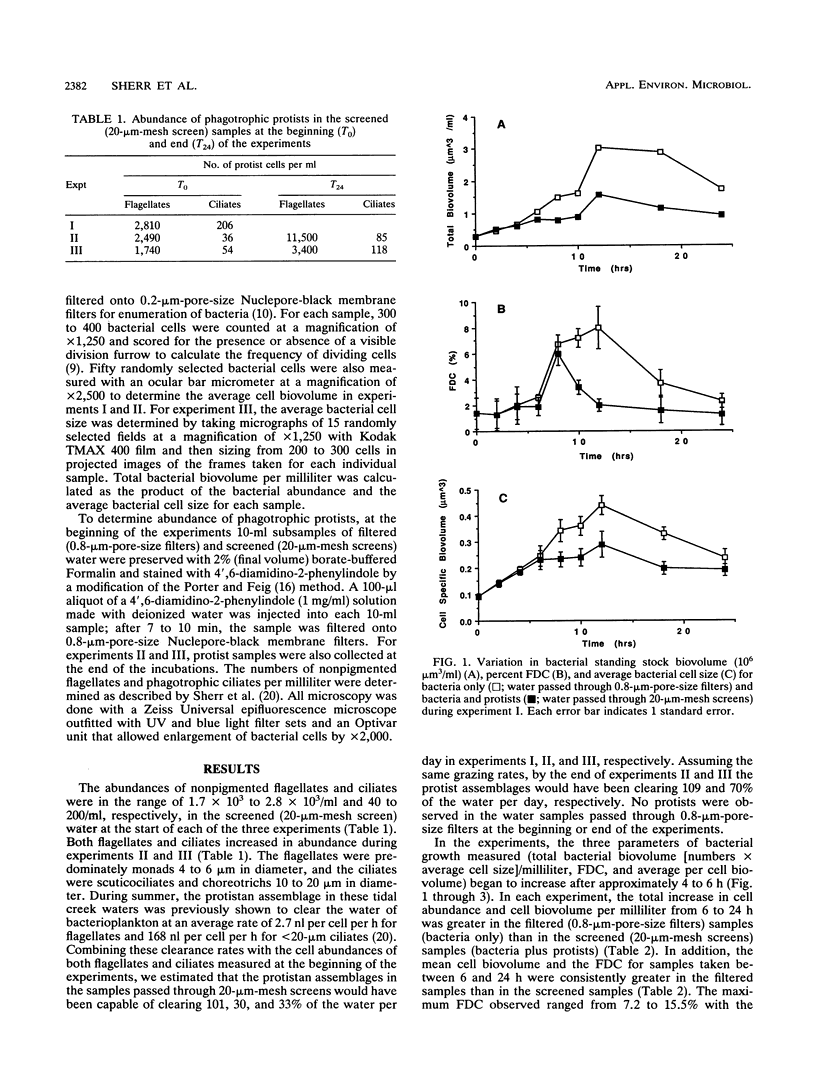
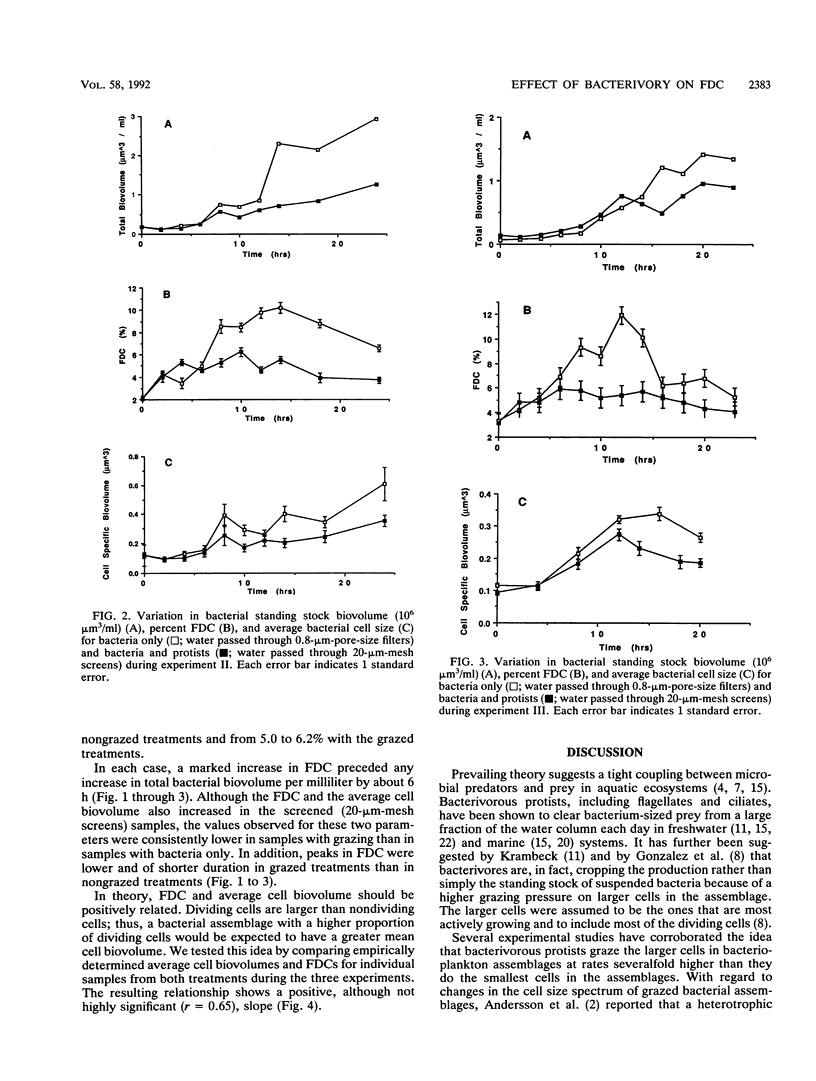
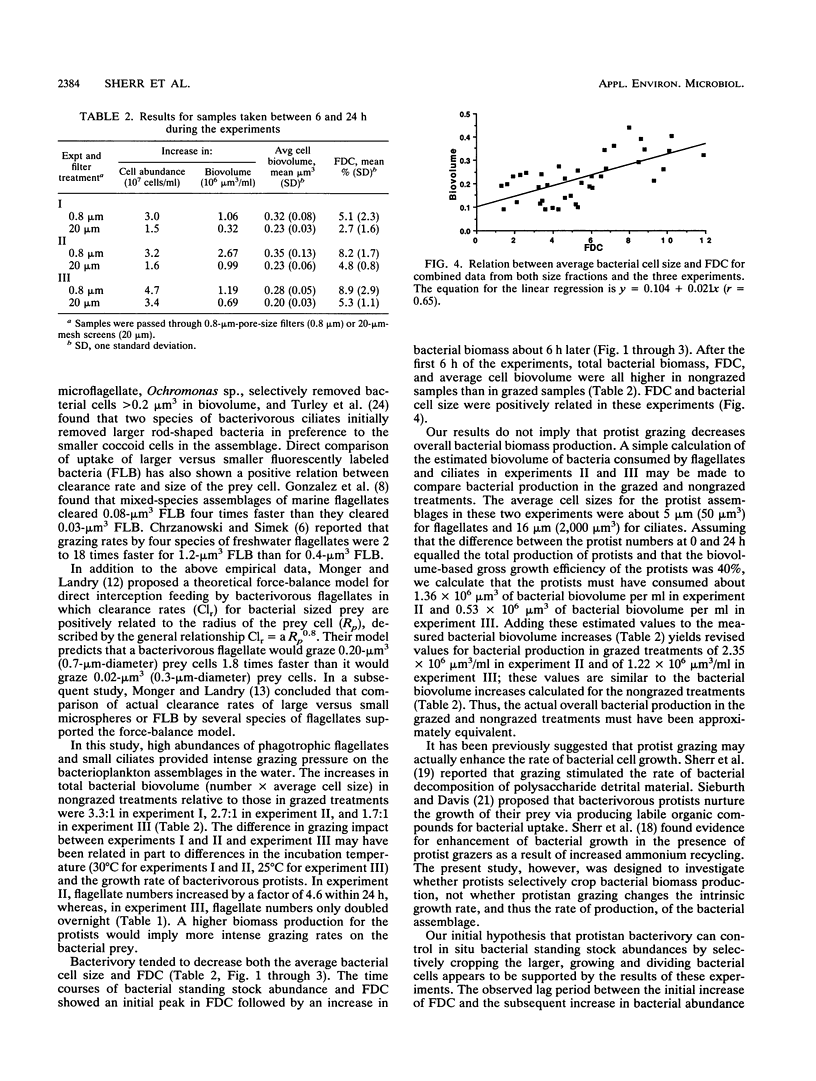
Grazing by phagotrophic flagellates and ciliates is a major source of mortality for bacterioplankton in both marine and freshwater systems. Recent studies have demonstrated a positive relationship between clearance rate and prey size for bacterivorous protists. We tested the idea that, by selectively grazing the larger (more actively growing or dividing) cells in a bacterial assemblage, protists control bacterial standing stock abundances by directly cropping bacterial production. Samples of estuarine water were passed through 0.8-μm-pore-size filters (bacteria only) or 20-μm-mesh screens (bacteria and bacterivorous protists) and placed in dialysis tubing suspended in 7 liters of unfiltered water. Changes in total bacterial biovolume per milliliter (bacterial biomass), frequency of dividing cells (FDC), and average per cell biovolume were followed over a period of 24 h. In three experiments, the FDC increased more rapidly and attained higher values in water passed through 0.8-μm-pore-size filters (average, 5.1 to 8.9%; maximum, 15.5%) compared with FDC values in water passed through 20-μm-mesh screens (average, 2.7 to 5.3%; maximum, 6.7%). Increases in bacterial biomass per milliliter lagged behind increases in FDC by about 4 to 6 h. Grazed bacterial assemblages were characterized by lower total biomasses and smaller average cell sizes compared with those of cells in nongrazed assemblages. We conclude that bacterivorous protists control bacterial standing stock abundances partly by preferentially removing dividing cells. Selective grazing of the more actively growing cells may also explain, in part, the ability of slow-growing cells to persist in bacterioplankton assemblages.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratbak G., Heldal M., Norland S., Thingstad T. F. Viruses as partners in spring bloom microbial trophodynamics. Appl Environ Microbiol. 1990 May;56(5):1400–1405. doi: 10.1128/aem.56.5.1400-1405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. M., Sherr E. B., Sherr B. F. Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol. 1990 Mar;56(3):583–589. doi: 10.1128/aem.56.3.583-589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström A., Larsson U., Hörstedt P., Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979 May;37(5):805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S. Y., Christian R. R. Frequency of dividing cells as an estimator of bacterial productivity. Appl Environ Microbiol. 1981 Jul;42(1):23–31. doi: 10.1128/aem.42.1.23-31.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


