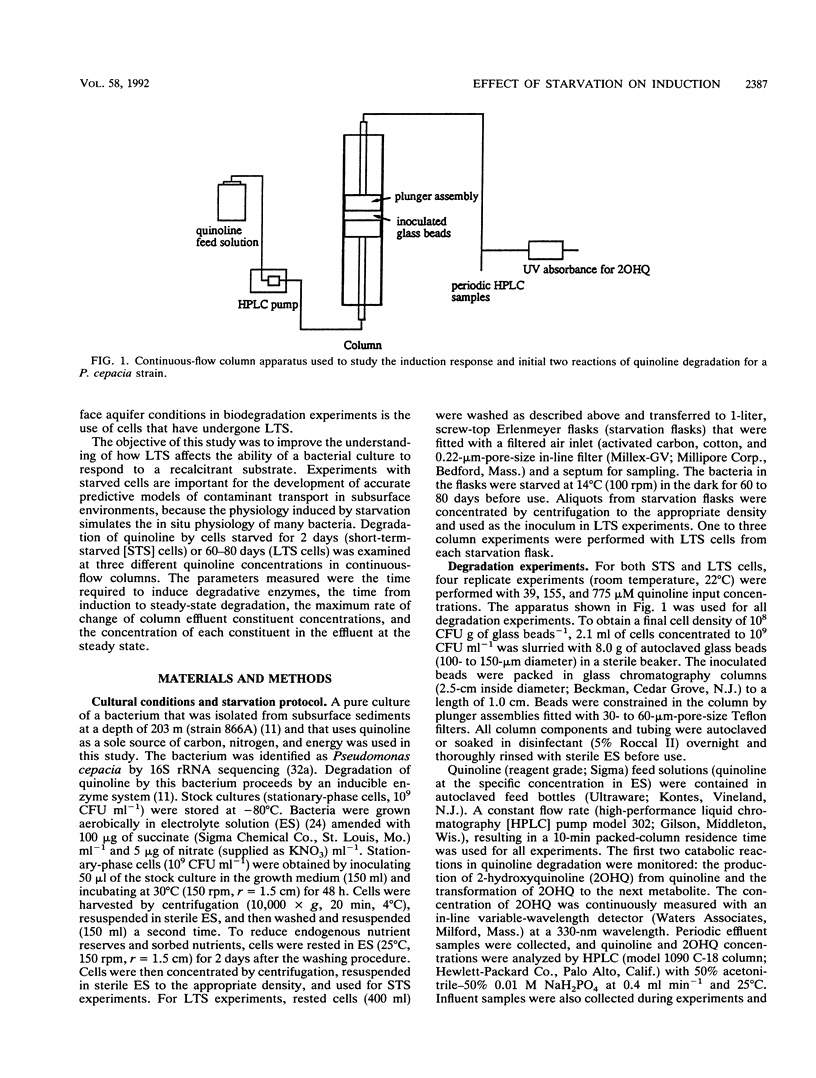
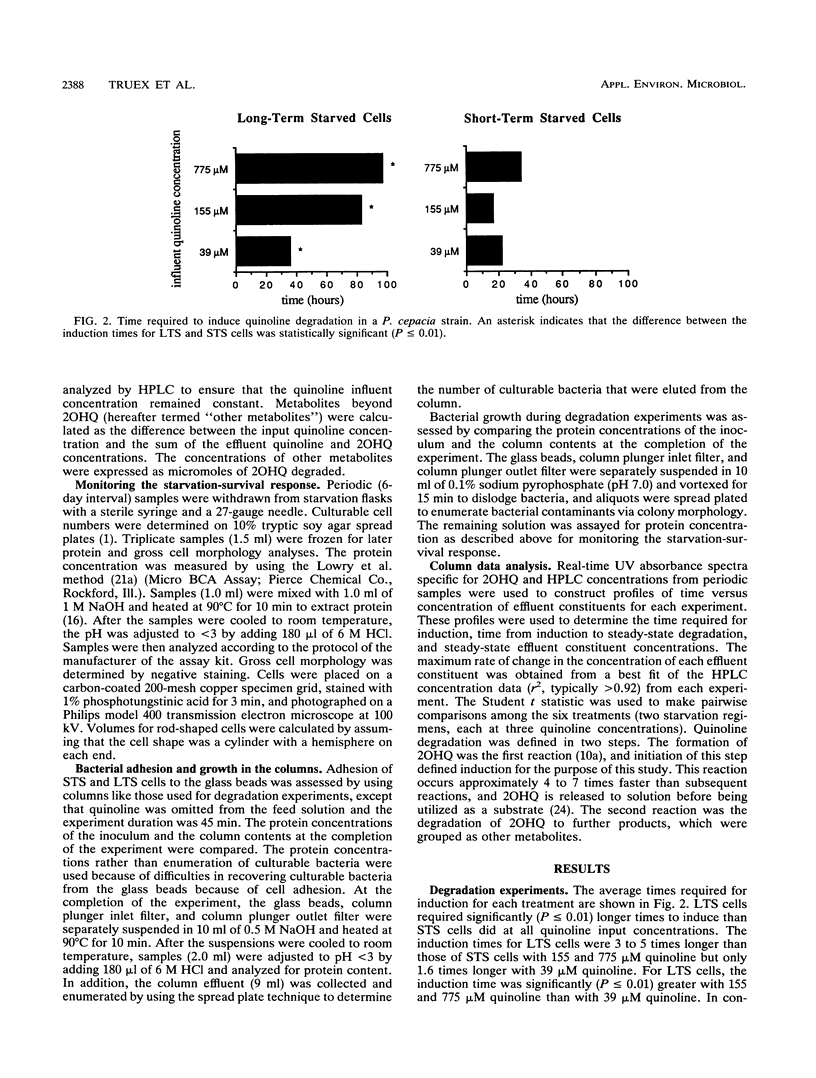
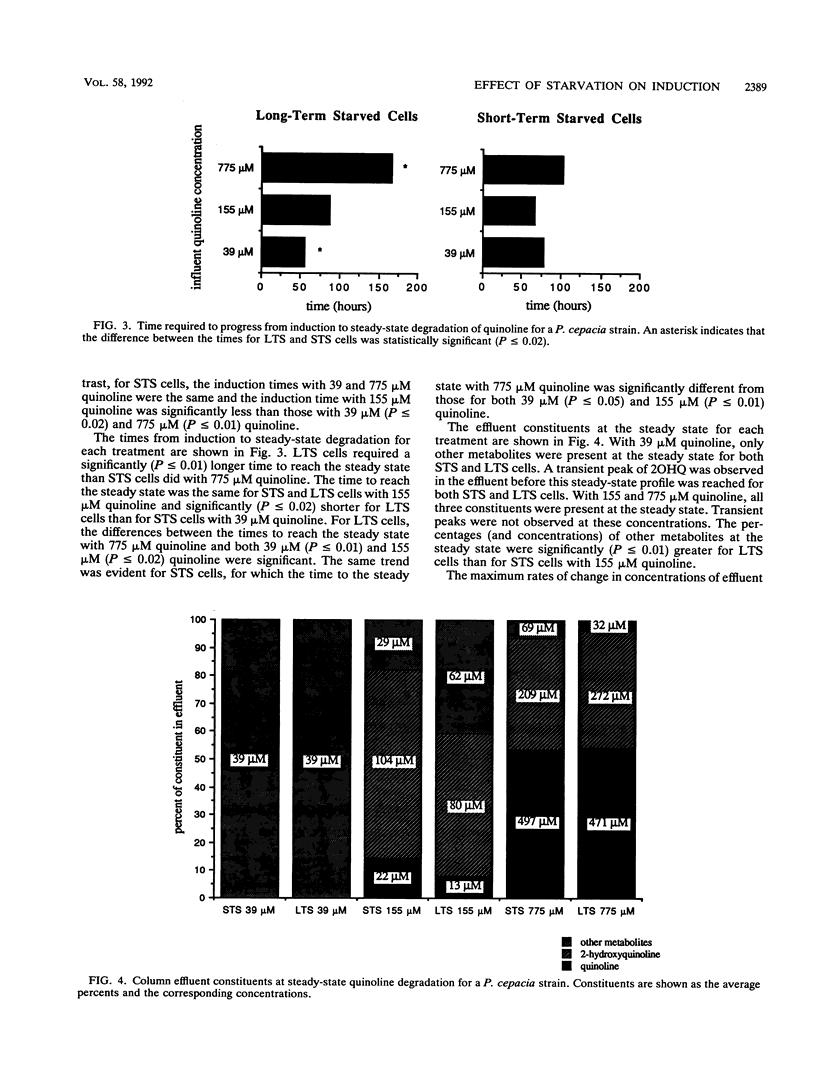
Abstract
Differences in the induction response and the initial two reactions of quinoline degradation between short-term (2 days)- and long-term (60 to 80 days)-starved cells of a subsurface Pseudomonas cepacia strain were examined by using continuous-flow columns. The ability of bacteria that are indigenous to oligotrophic environments to respond to a contaminant was assessed by using long-term starvation to induce a cell physiology that simulates the in situ physiology of the bacteria. With quinoline concentrations of 39 and 155 μM, long-term-starved cells converted quinoline to degradation products more efficiently than did short-term-starved cells. Quinoline concentrations of 155 μM and, to a greater extent, 775 μM had an inhibitory effect on induction in long-term-starved cells. However, only the length of the induction process was affected with these quinoline concentrations; degradation of quinoline at the steady state for long-term-starved cells was equal to or better than that for short-term-starved cells. The induction time for short-term-starved cells did not increase progressively with increasing quinoline concentration. Experiments with starved cells are important for the development of accurate predictive models of contaminant transport in the subsurface because starvation, which induces a cell physiology that simulates the in situ physiology of many bacteria, may affect the induction process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Morita R. Y. Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol. 1983 Mar;45(3):1109–1115. doi: 10.1128/aem.45.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Pauling C., Morita R. Y. Recovery from nutrient starvation by a marine Vibrio sp. Appl Environ Microbiol. 1983 May;45(5):1685–1690. doi: 10.1128/aem.45.5.1685-1690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Pauling C., Morita R. Y. Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol. 1983 Mar;45(3):1041–1048. doi: 10.1128/aem.45.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983 Apr;45(4):1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylen C. W., Ensign J. C. Intracellular substrates for endogenous metabolism during long-term starvation of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970 Sep;103(3):578–587. doi: 10.1128/jb.103.3.578-587.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman F. J., Denovan B. A., Hicks R. J., Fredrickson J. K. Isolation and characterization of quinoline-degrading bacteria from subsurface sediments. Appl Environ Microbiol. 1989 Apr;55(4):1029–1032. doi: 10.1128/aem.55.4.1029-1032.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapelle F. H., Lovley D. R. Rates of microbial metabolism in deep coastal plain aquifers. Appl Environ Microbiol. 1990 Jun;56(6):1865–1874. doi: 10.1128/aem.56.6.1865-1874.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign J. C. Long-term starvation survival of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970 Sep;103(3):569–577. doi: 10.1128/jb.103.3.569-577.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E. P., Zeyer J., Eicher P., Schwarzenbach R. P. Anaerobic degradation of alkylated benzenes in denitrifying laboratory aquifer columns. Appl Environ Microbiol. 1988 Feb;54(2):490–496. doi: 10.1128/aem.54.2.490-496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G., Morita R. Y. Starvation-Survival Physiological Studies of a Marine Pseudomonas sp. Appl Environ Microbiol. 1983 Apr;45(4):1206–1211. doi: 10.1128/aem.45.4.1206-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Moyer C. L., Morita R. Y. Effect of growth rate and starvation-survival on the viability and stability of a psychrophilic marine bacterium. Appl Environ Microbiol. 1989 May;55(5):1122–1127. doi: 10.1128/aem.55.5.1122-1127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. M., Parkinson D. Effect of starvation on survival of three bacterial isolates from an arctic soil. Can J Microbiol. 1978 Dec;24(12):1460–1467. doi: 10.1139/m78-235. [DOI] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Kuhn E. P., Schwarzenbach R. P. Rapid microbial mineralization of toluene and 1,3-dimethylbenzene in the absence of molecular oxygen. Appl Environ Microbiol. 1986 Oct;52(4):944–947. doi: 10.1128/aem.52.4.944-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



