Abstract
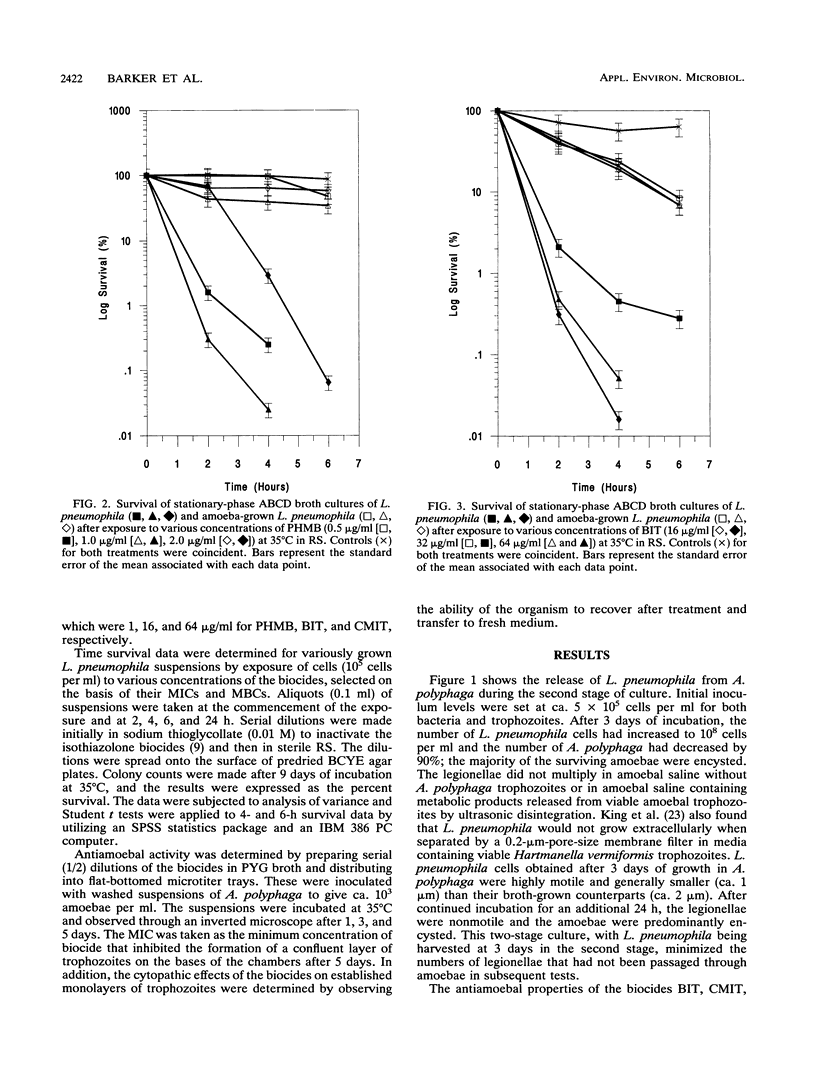
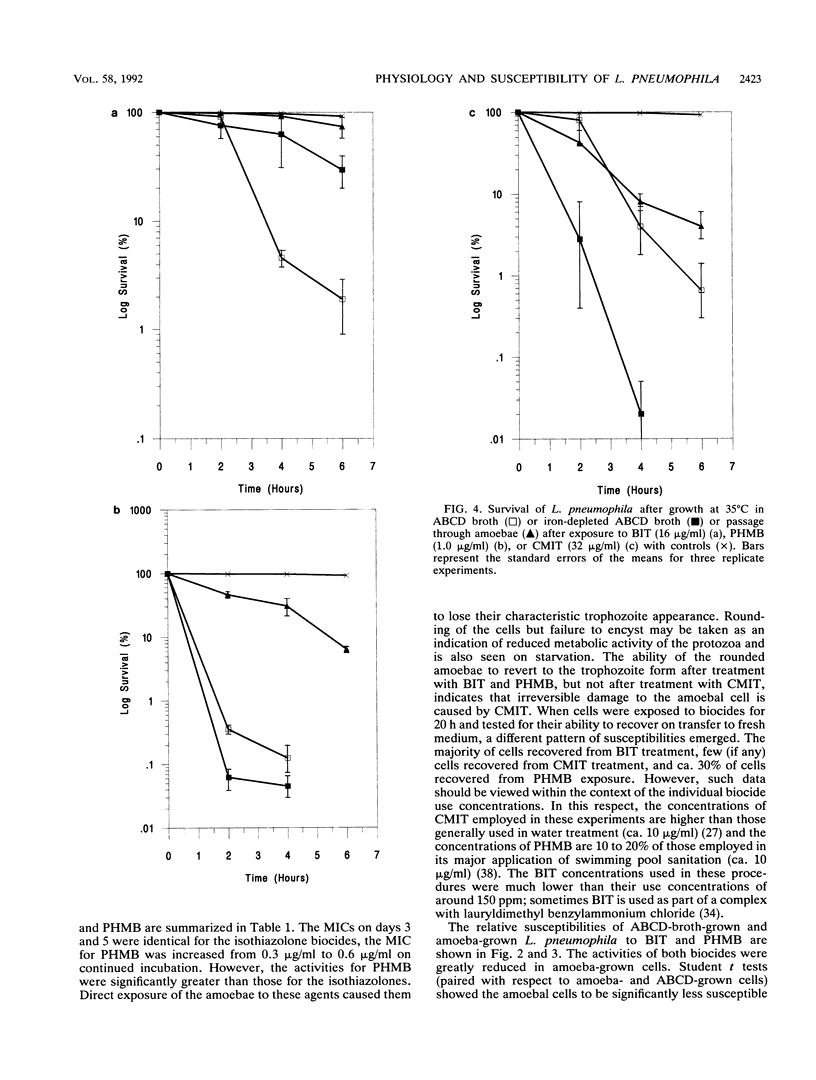
Survival studies were conducted on Legionella pneumophila cells that had been grown intracellularly in Acanthamoeba polyphaga and then exposed to polyhexamethylene biguanide (PHMB), benzisothiazolone (BIT), and 5-chloro-N-methylisothiazolone (CMIT). Susceptibilities were also determined for L. pneumophila grown under iron-sufficient and iron-depleted conditions. BIT was relatively ineffective against cells grown under iron depletion; in contrast, iron-depleted conditions increased the susceptibilities of cells to PHMB and CMIT. The activities of all three biocides were greatly reduced against L. pneumophila grown in amoebae. PHMB (1 x MIC) gave 99.99% reductions in viability for cultures grown in broth within 6 h and no detectable survivors at 24 h but only 90 and 99.9% killing at 6 h and 24 h, respectively, for cells grown in amoebae. The antimicrobial properties of the three biocides against A. polyphaga were also determined. The majority of amoebae recovered from BIT treatment, but few, if any, survived CMIT treatment or exposure to PHMB. This study not only shows the profound effect that intra-amoebal growth has on the physiological status and antimicrobial susceptibility of L. pneumophila but also reveals PHMB to be a potential biocide for effective water treatment. In this respect, PHMB has significant activity, below its recommended use concentrations, against both the host amoeba and L. pneumophila.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J., Till D. H. Survival of Legionella pneumophila. Med Lab Sci. 1986 Oct;43(4):388–389. [PubMed] [Google Scholar]
- Brown M. R., Allison D. G., Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988 Dec;22(6):777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Collier P. J., Gilbert P. Influence of growth rate on susceptibility to antimicrobial agents: modification of the cell envelope and batch and continuous culture studies. Antimicrob Agents Chemother. 1990 Sep;34(9):1623–1628. doi: 10.1128/aac.34.9.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Williams P. Influence of substrate limitation and growth phase on sensitivity to antimicrobial agents. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):7–14. doi: 10.1093/jac/15.suppl_a.7. [DOI] [PubMed] [Google Scholar]
- Broxton P., Woodcock P. M., Heatley F., Gilbert P. Interaction of some polyhexamethylene biguanides and membrane phospholipids in Escherichia coli. J Appl Bacteriol. 1984 Aug;57(1):115–124. doi: 10.1111/j.1365-2672.1984.tb02363.x. [DOI] [PubMed] [Google Scholar]
- Collier P. J., Ramsey A. J., Austin P., Gilbert P. Growth inhibitory and biocidal activity of some isothiazolone biocides. J Appl Bacteriol. 1990 Oct;69(4):569–577. doi: 10.1111/j.1365-2672.1990.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Edelstein P. H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981 Sep;14(3):298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsmore R. Biocidal control of legionellae. Isr J Med Sci. 1986 Sep;22(9):647–654. [PubMed] [Google Scholar]
- Gilbert P., Collier P. J., Brown M. R. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990 Oct;34(10):1865–1868. doi: 10.1128/aac.34.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Pemberton D., Wilkinson D. E. Barrier properties of the gram-negative cell envelope towards high molecular weight polyhexamethylene biguanides. J Appl Bacteriol. 1990 Oct;69(4):585–592. doi: 10.1111/j.1365-2672.1990.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Joly J. R., McKinney R. M., Tobin J. O., Bibb W. F., Watkins I. D., Ramsay D. Development of a standardized subgrouping scheme for Legionella pneumophila serogroup 1 using monoclonal antibodies. J Clin Microbiol. 1986 Apr;23(4):768–771. doi: 10.1128/jcm.23.4.768-771.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Shand G. H., Ward K. H. Media for study of growth kinetics and envelope properties of iron-deprived bacteria. J Clin Microbiol. 1987 May;25(5):849–855. doi: 10.1128/jcm.25.5.849-855.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilvington S., Price J. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Bacteriol. 1990 May;68(5):519–525. doi: 10.1111/j.1365-2672.1990.tb02904.x. [DOI] [PubMed] [Google Scholar]
- King C. H., Fields B. S., Shotts E. B., Jr, White E. H. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect Immun. 1991 Mar;59(3):758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J. B., Bartlett C. L., Newton U. A., White R. A., Jones N. L. Legionella pneumophila in cooling water systems. Report of a survey of cooling towers in London and a pilot trial of selected biocides. J Hyg (Lond) 1982 Jun;88(3):369–381. doi: 10.1017/s0022172400070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. V., West A. A. Survival and growth of Legionella species in the environment. Soc Appl Bacteriol Symp Ser. 1991;20:121S–129S. [PubMed] [Google Scholar]
- Monte W. C., Ashoor S. H., Lewis B. J. Mutagenicity of two non-formaldehyde-forming antimicrobial agents. Food Chem Toxicol. 1983 Oct;21(5):695–696. doi: 10.1016/0278-6915(83)90196-5. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Weaver R. E., Dees S. B., Cherry W. B. Cellular fatty acid composition of isolates from Legionnaires disease. J Clin Microbiol. 1977 Aug;6(2):140–143. doi: 10.1128/jcm.6.2.140-143.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Hoffman P. S., Malcolm G. B., Benson R. F., Franzus M. J. Role of keto acids and reduced-oxygen-scavenging enzymes in the growth of Legionella species. J Clin Microbiol. 1986 Jan;23(1):33–42. doi: 10.1128/jcm.23.1.33-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham T. J. Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci. 1986 Sep;22(9):678–689. [PubMed] [Google Scholar]
- Rowbotham T. J. Isolation of Legionella pneumophila from clinical specimens via amoebae, and the interaction of those and other isolates with amoebae. J Clin Pathol. 1983 Sep;36(9):978–986. doi: 10.1136/jcp.36.9.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaliy P., Thompson T. A., Gorman G. W., Morris G. K., McEachern H. V., Mackel D. C. Laboratory studies of disinfectants against Legionella pneumophila. Appl Environ Microbiol. 1980 Oct;40(4):697–700. doi: 10.1128/aem.40.4.697-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F., Surgot M., Bornstein N., Paucod J. C., Marmet D., Isoard P., Fleurette J. Relationship between free amoeba and Legionella: studies in vitro and in vivo. Zentralbl Bakteriol. 1990 Mar;272(3):265–275. doi: 10.1016/s0934-8840(11)80027-7. [DOI] [PubMed] [Google Scholar]
- Weaver J. E., Cardin C. W., Maibach H. I. Dose-response assessments of Kathon biocide (I). Diagnostic use and diagnostic threshold patch testing with sensitized humans. Contact Dermatitis. 1985 Mar;12(3):141–145. doi: 10.1111/j.1600-0536.1985.tb01083.x. [DOI] [PubMed] [Google Scholar]


