Abstract
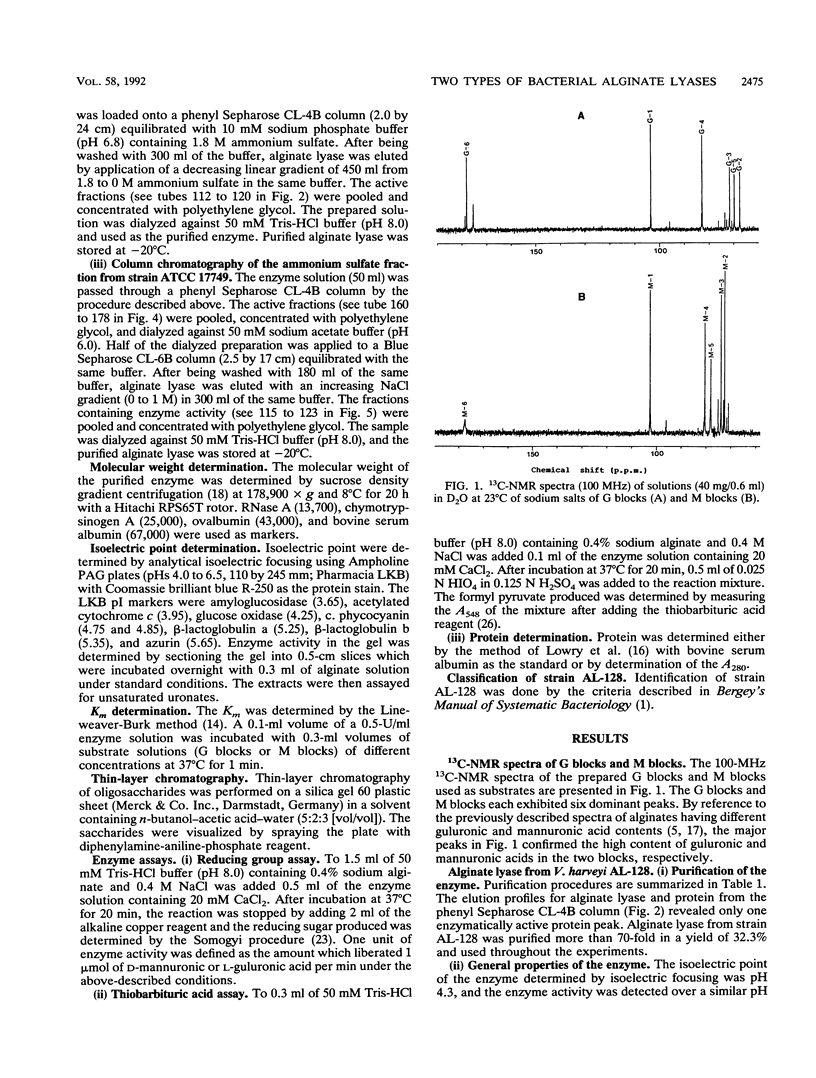
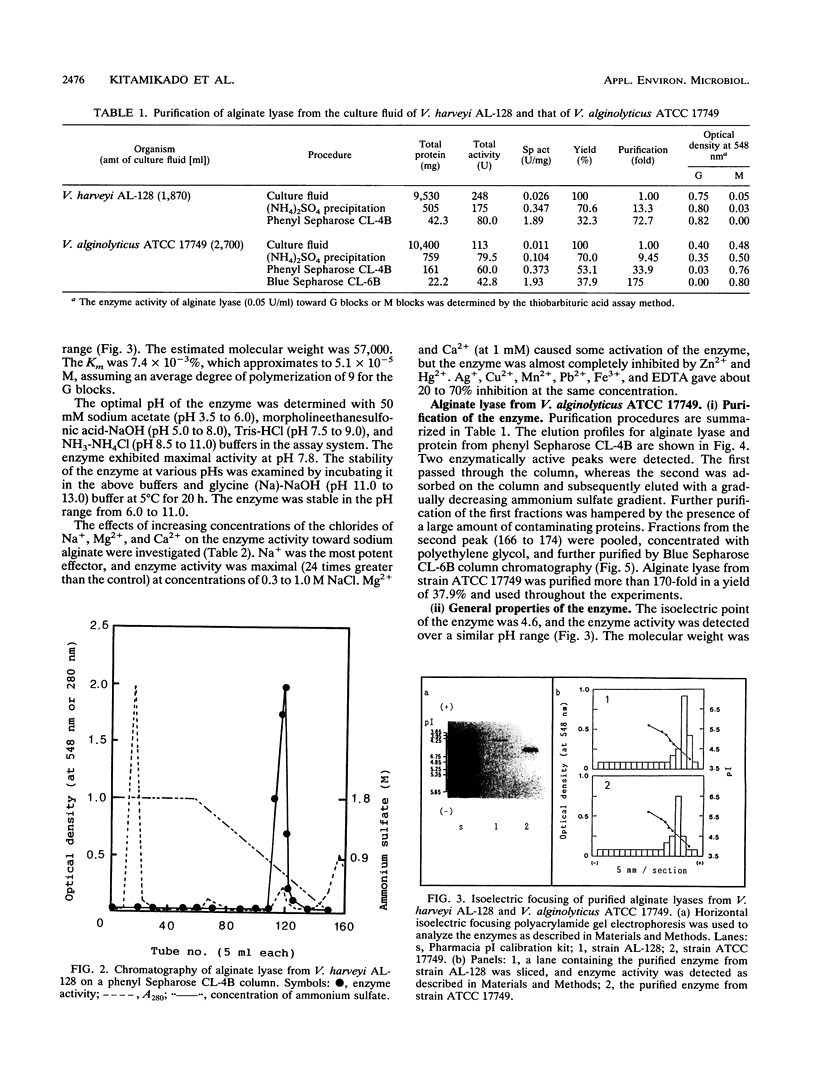
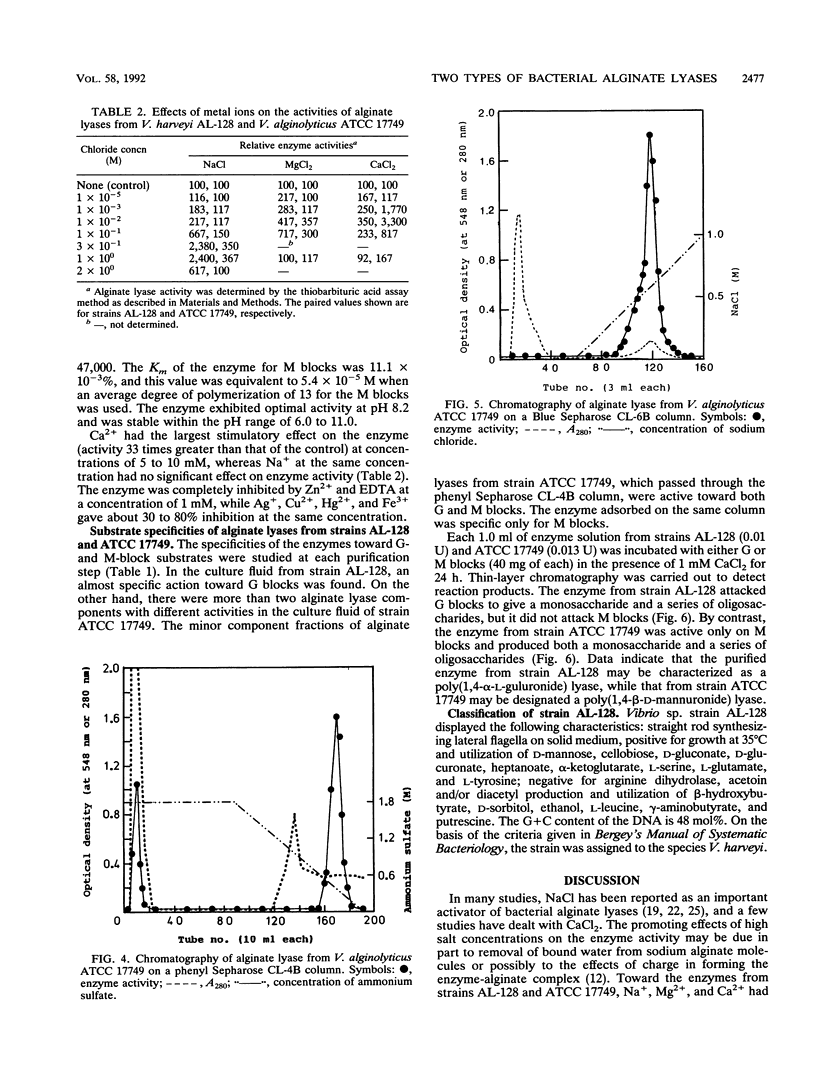
The extracellular alginate lyases were purified from Vibrio harveyi AL-128 and V. alginolyticus ATCC 17749. The former enzyme appears to be specific for alpha-1,4 bonds involving L-guluronate units in alginate, whereas the latter exhibits specificity for beta-1,4 bonds involving D-mannuronate units. The molecular weights of the enzymes were estimated to be 57,000 and 47,000, and they had isoelectric points of 4.3 and 4.6, respectively. The enzyme from strain AL-128 was most active at NaCl concentrations of 0.3 to 1.0 M. Optimum activity of the enzyme from strain ATCC 17749 was found in the presence of 5 to 10 mM CaCl2.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J., Turvey J. R. Isolation of poly-alpha-L-guluronate lyase from Klebsiella aerogenes. Carbohydr Res. 1977 Aug;57:163–171. doi: 10.1016/s0008-6215(00)81928-x. [DOI] [PubMed] [Google Scholar]
- Doubet R. S., Quatrano R. S. Isolation of marine bacteria capable of producing specific lyases for alginate degradation. Appl Environ Microbiol. 1982 Sep;44(3):754–756. doi: 10.1128/aem.44.3.754-756.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Doubet R. S., Ram J. Alginase enzyme production by Bacillus circulans. Appl Environ Microbiol. 1984 Apr;47(4):704–709. doi: 10.1128/aem.47.4.704-709.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamikado M., Yamaguchi K., Tseng C. H., Okabe B. Method designed to detect alginate-degrading bacteria. Appl Environ Microbiol. 1990 Sep;56(9):2939–2940. doi: 10.1128/aem.56.9.2939-2940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linker A., Evans L. R. Isolation and characterization of an alginase from mucoid strains of Pseudomonas aeruginosa. J Bacteriol. 1984 Sep;159(3):958–964. doi: 10.1128/jb.159.3.958-964.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Min K. H., Sasaki S. F., Kashiwabara Y., Suzuki H., Nisizawa K. Multiple components of endo-polyguluronide lyase of Pseudomonas sp. J Biochem. 1977 Mar;81(3):539–546. doi: 10.1093/oxfordjournals.jbchem.a131488. [DOI] [PubMed] [Google Scholar]
- Nakada H. I., Sweeny P. C. Alginic acid degradation by eliminases from abalone hepatopancreas. J Biol Chem. 1967 Mar 10;242(5):845–851. [PubMed] [Google Scholar]
- Nisizawa K., Fujibayashi S., Kashiwabara Y. Alginate lyases in the hepatopancreas of a marine mollusc, Dolabella auricula Solander. J Biochem. 1968 Jul;64(1):25–37. doi: 10.1093/oxfordjournals.jbchem.a128859. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Stevens R. A., Levin R. E. Purification and characteristics of an alginase from Alginovibrio aquatilis. Appl Environ Microbiol. 1977 May;33(5):1156–1161. doi: 10.1128/aem.33.5.1156-1161.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]




