Abstract
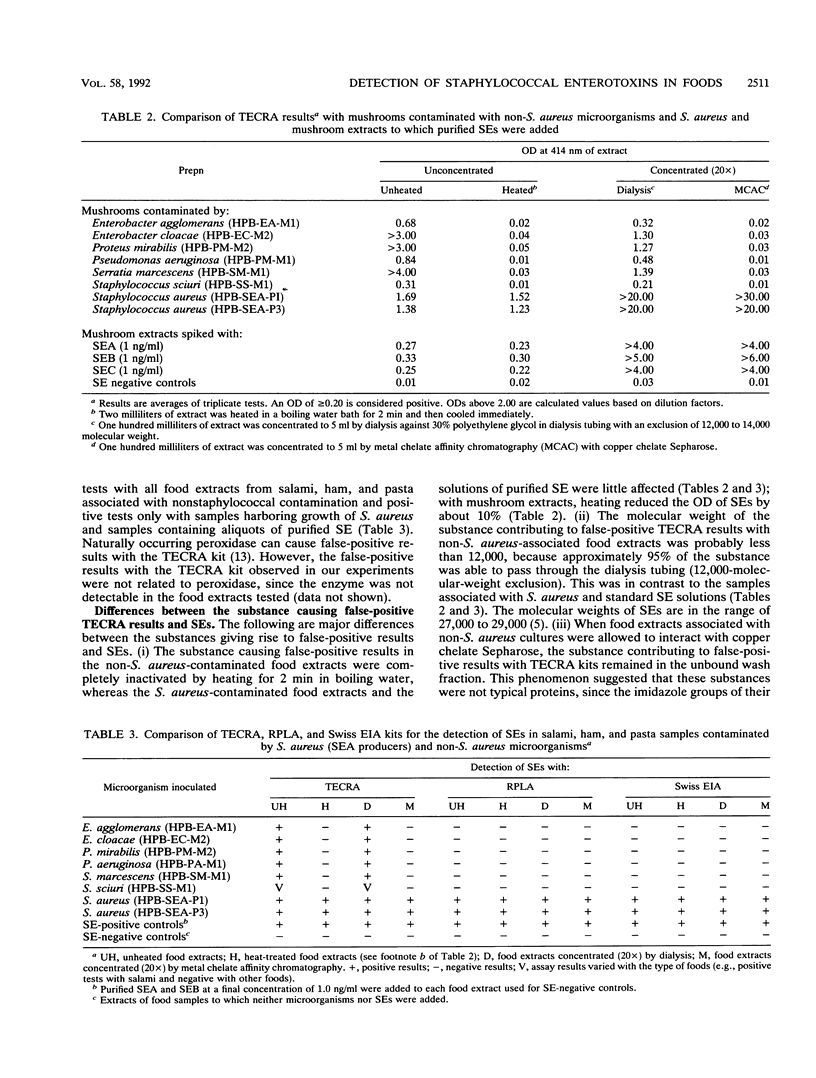
A staphylococcal enterotoxin visual immunoassay kit (TECRA) has recently become commercially available. Since the kit is an enzyme-linked immunosorbent assay system equipped with polyvalent antisera against staphylococcal enterotoxin types A to E (SEA to SEE) and the test is simple and rapid to perform (4 h), it has been widely used for screening purposes. In this study, the sensitivity of the kit for detection of SEA, SEB, and SEC in ham, cheese, and mushrooms was similar to those of kits based on an enzyme immunoassay and reversed passive latex agglutination: 0.75 to 1.0 ng of SEA per ml, 0.5 to 0.75 ng of SEB per ml, and 1.0 to 1.25 ng of SEC per ml. However, the TECRA kit showed nonspecific reactions with food samples contaminated by microorganisms other than Staphylococcus aureus, such as Enterobacter agglomerans, Enterobacter cloacae, Proteus mirabilis, Pseudomonas aeruginosa, and Serratia marcescens. The substance contributing to the false-positive results differed from true staphylococcal enterotoxins in that it was (i) heat labile (completely inactivated by heating for 2 min at 100 degrees C, whereas true staphylococcal enterotoxins were inactivated by about 10% with this treatment), (ii) lower in molecular weight than staphylococcal enterotoxins, and (iii) not bound to a copper chelate Sepharose gel (all of the substance remained in the unbound wash fraction, whereas staphylococcal enterotoxins were quantitatively bound to the gel). The problem of false-positive results with the TECRA kit could be resolved by heat treatment (2 min at 100 degrees C) or by cleanup procedures involving metal chelate affinity chromatography with copper chelate Sepharose for 4 h before use of the TECRA kit.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesiyun A. A., Tatini S. R., Hoover D. G. Production of enterotoxin(s) by Staphylococcus hyicus. Vet Microbiol. 1984 Sep;9(5):487–495. doi: 10.1016/0378-1135(84)90069-5. [DOI] [PubMed] [Google Scholar]
- Bankes P., Rose S. A. Rapid detection of staphylococcal enterotoxins in foods with a modification of the reversed passive latex agglutination assay. J Appl Bacteriol. 1989 Oct;67(4):395–399. doi: 10.1111/j.1365-2672.1989.tb02509.x. [DOI] [PubMed] [Google Scholar]
- Bergdoll M. S. Analytical methods for Staphylococcus aureus. Int J Food Microbiol. 1990 Mar;10(2):91–99. doi: 10.1016/0168-1605(90)90058-d. [DOI] [PubMed] [Google Scholar]
- Collins W. S., Johnson A. D., Metzger J. F., Bennett R. W. Rapid solid-phase radioimmunoassay for staphylococcal enterotoxin A. Appl Microbiol. 1973 May;25(5):774–777. doi: 10.1128/am.25.5.774-777.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson M. L., Hinds M. W., Bernstein R. S., Bergdoll M. S. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int J Food Microbiol. 1988 Dec 31;7(4):311–316. doi: 10.1016/0168-1605(88)90057-8. [DOI] [PubMed] [Google Scholar]
- Ewald S. Evaluation of enzyme-linked immunosorbent assay (ELISA) for detection of staphylococcal enterotoxin in foods. Int J Food Microbiol. 1988 Mar;6(2):141–153. doi: 10.1016/0168-1605(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Fey H., Pfister H., Rüegg O. Comparative evaluation of different enzyme-linked immunosorbent assay systems for the detection of staphylococcal enterotoxins A, B, C, and D. J Clin Microbiol. 1984 Jan;19(1):34–38. doi: 10.1128/jcm.19.1.34-38.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed R. C., Evenson M. L., Reiser R. F., Bergdoll M. S. Enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. Appl Environ Microbiol. 1982 Dec;44(6):1349–1355. doi: 10.1128/aem.44.6.1349-1355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S., Tokuno H., Ogawa H., Sasaki M., Kishimoto T., Kawano J., Shimizu A., Kimura S. Enterotoxigenicity of Staphylococcus intermedius strains isolated from dogs. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(2-3):360–367. doi: 10.1016/s0176-6724(84)80054-1. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Bukovic J. A., Kauffmann P. E. Staphylococcal enterotoxins A and B: solid-phase radioimmunoassay in food. Appl Microbiol. 1973 Sep;26(3):309–313. doi: 10.1128/am.26.3.309-313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLandsborough L., Tatini S. R. A 6 h microslide immunodiffusion assay for confirmed detection of staphylococcal enterotoxins. Lett Appl Microbiol. 1991 Mar;12(3):81–84. doi: 10.1111/j.1472-765x.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Park C. E., Szabo R. Evaluation of the reversed passive latex agglutination (RPLA) test kits for detection of staphylococcal enterotoxins A, B, C, and D in foods. Can J Microbiol. 1986 Sep;32(9):723–727. doi: 10.1139/m86-131. [DOI] [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Robern H., Stavric S., Dickie N. The application of QAE-Sephadex for the purification of two staphylococcal enterotoxins. I. Purification of enterotoxin C2. Biochim Biophys Acta. 1975 May 30;393(1):148–158. doi: 10.1016/0005-2795(75)90225-1. [DOI] [PubMed] [Google Scholar]
- Wieneke A. A. Comparison of four kits for the detection of staphylococcal enterotoxin in foods from outbreaks of food poisoning. Int J Food Microbiol. 1991 Dec;14(3-4):305–312. doi: 10.1016/0168-1605(91)90122-6. [DOI] [PubMed] [Google Scholar]
- Wieneke A. A. The detection of enterotoxin and toxic shock syndrome toxin-1 production by strains of Staphylococcus aureus with commercial RPLA kits. Int J Food Microbiol. 1988 Aug;7(1):25–30. doi: 10.1016/0168-1605(88)90069-4. [DOI] [PubMed] [Google Scholar]


