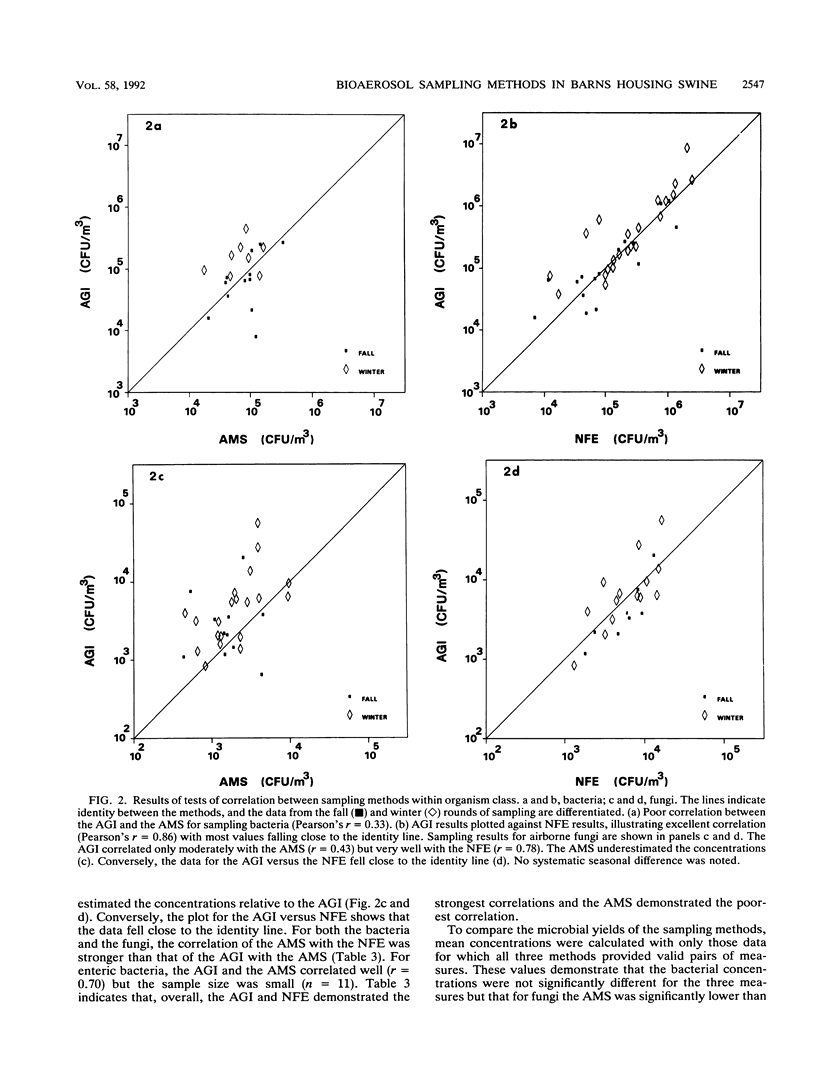
Abstract
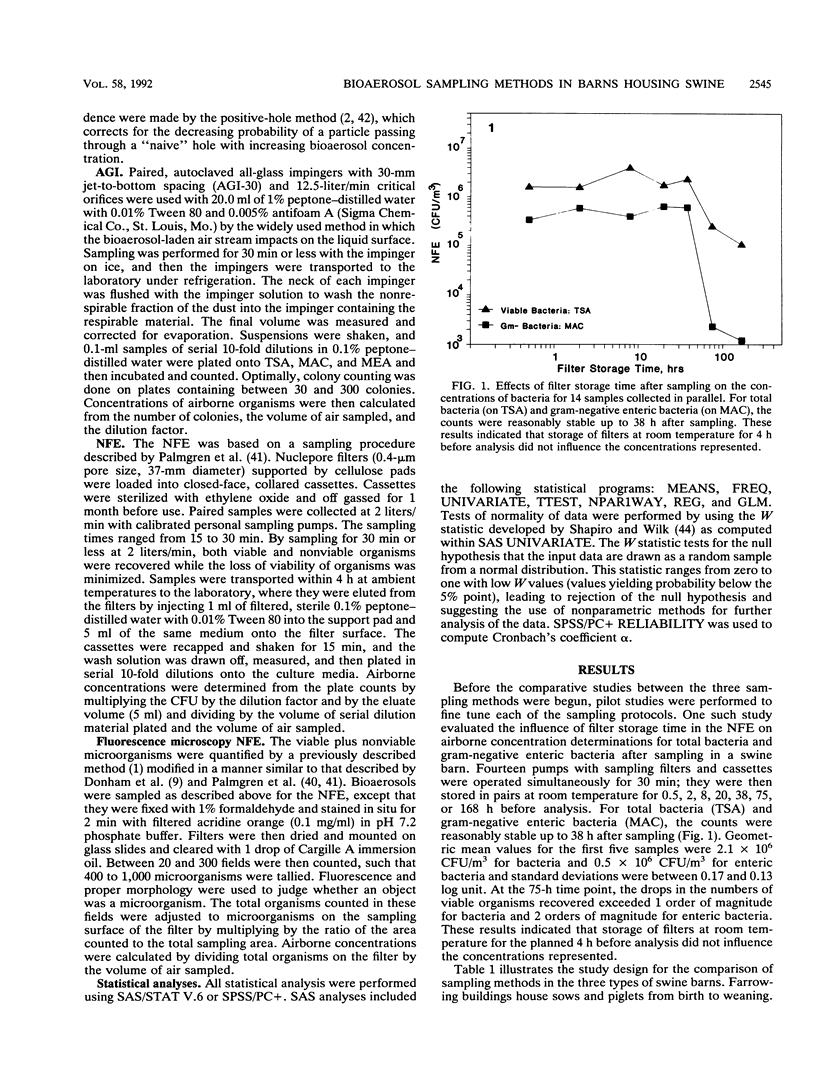
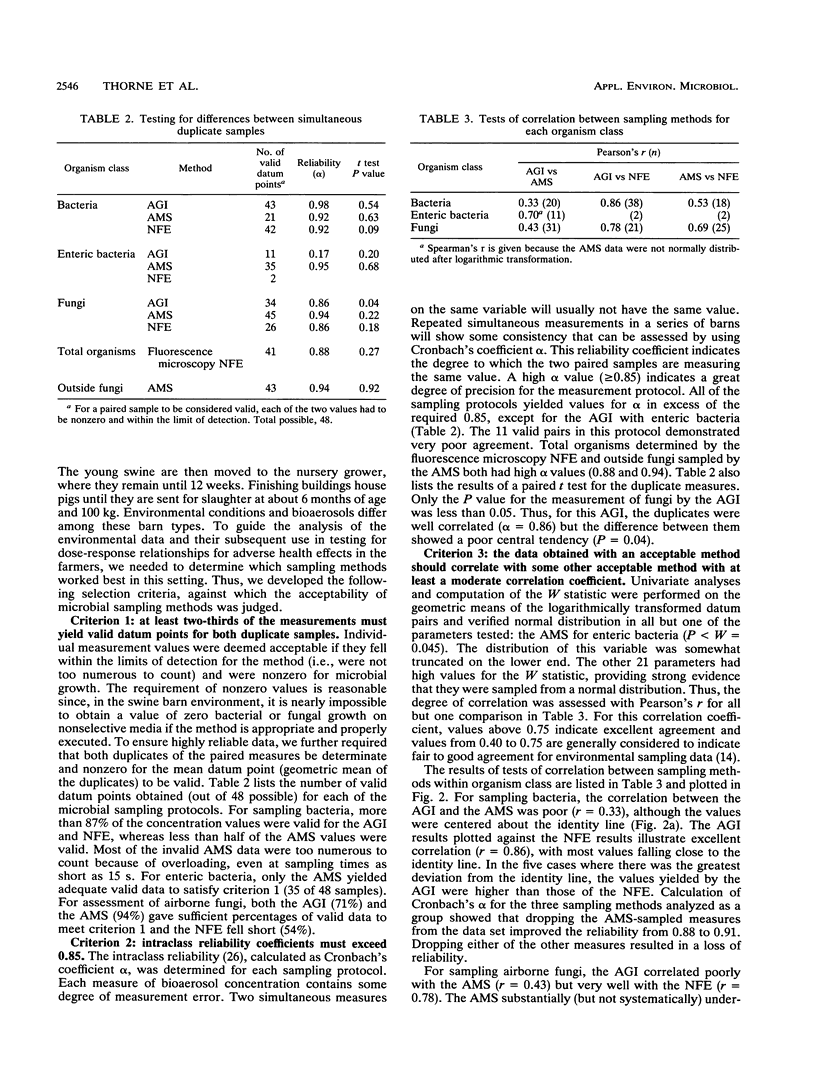
The air in livestock buildings contains bioaerosol levels that are sufficiently high to cause adverse health effects in animals and workers. These bioaerosols are complex mixtures of live and dead microorganisms and their products as well as other aeroallergens. The effectiveness of sampling methods used for quantifying the very high concentrations of microorganisms in these environments has not been well studied. To facilitate an accurate assessment of respiratory hazards from viable organisms in agricultural environments, three bioaerosol sampling methods were investigated: the Andersen microbial sampler method (AMS), the all-glass impinger method (AGI), and the Nuclepore filtration-elution method (NFE). These methods were studied in a parallel fashion in 24 swine confinement buildings. Measurements were taken in two seasons with three types of culture media in duplicate to assess total bacteria, gram-negative enteric bacteria, and total fungi. Methods were analyzed for the proportion of samples yielding data within the limits of detection, intraclass reliability, and correlation between methods. For sampling viable bacteria, the AMS had a poor data yield because of overloading and demonstrated weak correlation with the AGI. Conversely, the AGI and NFE gave sufficient numbers of valid data points (90%), yielded high intraclass reliabilities (alpha greater than or equal to 0.92), and were highly correlated with each other (r = 0.86). The AGI and the NFE were suitable methods for assessing bacteria in this environment, but the AMS was not. The AMS was the only method that consistently recovered enteric bacteria (73% data yield). For sampling fungi, the AGI and AMS both yielded sufficient data and all three methods demonstrated high intraclass reliability.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN A. A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J Bacteriol. 1958 Nov;76(5):471–484. doi: 10.1128/jb.76.5.471-484.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner M. P., Stetzenbach L. D. Evaluation of Four Aerobiological Sampling Methods for the Retrieval of Aerosolized Pseudomonas syringae. Appl Environ Microbiol. 1991 Apr;57(4):1268–1270. doi: 10.1128/aem.57.4.1268-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S., Rylander R., Larsson L. Airborne bacteria, endotoxin and fungi in dust in poultry and swine confinement buildings. Am Ind Hyg Assoc J. 1983 Jul;44(7):537–541. doi: 10.1080/15298668391405265. [DOI] [PubMed] [Google Scholar]
- Crook B., Robertson J. F., Glass S. A., Botheroyd E. M., Lacey J., Topping M. D. Airborne dust, ammonia, microorganisms, and antigens in pig confinement houses and the respiratory health of exposed farm workers. Am Ind Hyg Assoc J. 1991 Jul;52(7):271–279. doi: 10.1080/15298669191364721. [DOI] [PubMed] [Google Scholar]
- Donham K. J. Hazardous agents in agricultural dusts and methods of evaluation. Am J Ind Med. 1986;10(3):205–220. doi: 10.1002/ajim.4700100305. [DOI] [PubMed] [Google Scholar]
- Donham K. J., Popendorf W., Palmgren U., Larsson L. Characterization of dusts collected from swine confinement buildings. Am J Ind Med. 1986;10(3):294–297. doi: 10.1002/ajim.4700100318. [DOI] [PubMed] [Google Scholar]
- Donham K. J., Scallon L. J., Popendorf W., Treuhaft M. W., Roberts R. C. Characterization of dusts collected from swine confinement buildings. Am Ind Hyg Assoc J. 1986 Jul;47(7):404–410. doi: 10.1080/15298668691389955. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz J., Olenchock S. A., Sorenson W. G., Gerencser V. F., May J. J., Pratt D. S., Robinson V. A. Levels of bacteria, fungi, and endotoxin in bulk and aerosolized corn silage. Appl Environ Microbiol. 1989 May;55(5):1093–1099. doi: 10.1128/aem.55.5.1093-1099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields N. D., Oxborrow G. S., Puleo J. R., Herring C. M. Evaluation fo membrane filter field monitors for microbiological air sampling. Appl Microbiol. 1974 Mar;27(3):517–520. doi: 10.1128/am.27.3.517-520.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heederik D., Brouwer R., Biersteker K., Boleij J. S. Relationship of airborne endotoxin and bacteria levels in pig farms with the lung function and respiratory symptoms of farmers. Int Arch Occup Environ Health. 1991;62(8):595–601. doi: 10.1007/BF00381114. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. R., Butchart A. M., Kgamphe S. J. A comparison of sampling methods for airborne bacteria. Environ Res. 1978 Jul;16(1-3):279–284. doi: 10.1016/0013-9351(78)90162-7. [DOI] [PubMed] [Google Scholar]
- Jones W., Morring K., Morey P., Sorenson W. Evaluation of the Andersen viable impactor for single stage sampling. Am Ind Hyg Assoc J. 1985 May;46(5):294–298. doi: 10.1080/15298668591394833. [DOI] [PubMed] [Google Scholar]
- Jones W., Morring K., Olenchock S. A., Williams T., Hickey J. Environmental study of poultry confinement buildings. Am Ind Hyg Assoc J. 1984 Nov;45(11):760–766. [PubMed] [Google Scholar]
- Lacey J., Crook B. Fungal and actinomycete spores as pollutants of the workplace and occupational allergens. Ann Occup Hyg. 1988;32(4):515–533. doi: 10.1093/annhyg/32.4.515. [DOI] [PubMed] [Google Scholar]
- Lembke L. L., Kniseley R. N., van Nostrand R. C., Hale M. D. Precision of the all-glass impinger and the andersen microbial impactor for air sampling in solid-waste handling facilities. Appl Environ Microbiol. 1981 Aug;42(2):222–225. doi: 10.1128/aem.42.2.222-225.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm I. M. Comparison of methods for quantitative determinations of airborne bacteria and evaluation of total viable counts. Appl Environ Microbiol. 1982 Jul;44(1):179–183. doi: 10.1128/aem.44.1.179-183.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY K. R. CALIBRATION OF A MODIFIED ANDERSEN BACTERIAL AEROSOL SAMPLER. Appl Microbiol. 1964 Jan;12:37–43. doi: 10.1128/am.12.1.37-43.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY K. R., HARPER G. J. The efficiency of various liquid impinger samplers in bacterial aerosols. Br J Ind Med. 1957 Oct;14(4):287–297. doi: 10.1136/oem.14.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher J. M., First M. W. Personal air samplers for measuring occupational exposures to biological hazards. Am Ind Hyg Assoc J. 1984 Feb;45(2):76–83. doi: 10.1080/15298668491399406. [DOI] [PubMed] [Google Scholar]
- Macher J. M. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am Ind Hyg Assoc J. 1989 Nov;50(11):561–568. doi: 10.1080/15298668991375164. [DOI] [PubMed] [Google Scholar]
- Malmberg P., Rask-Andersen A., Palmgren U., Höglund S., Kolmodin-Hedman B., Stålenheim G. Exposure to microorganisms, febrile and airway-obstructive symptoms, immune status and lung function of Swedish farmers. Scand J Work Environ Health. 1985 Aug;11(4):287–293. doi: 10.5271/sjweh.2220. [DOI] [PubMed] [Google Scholar]
- Marthi B., Fieland V. P., Walter M., Seidler R. J. Survival of bacteria during aerosolization. Appl Environ Microbiol. 1990 Nov;56(11):3463–3467. doi: 10.1128/aem.56.11.3463-3467.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant J. A. Agricultural exposures to organic dusts. Occup Med. 1987 Apr-Jun;2(2):409–425. [PubMed] [Google Scholar]
- Palmgren U., Ström G., Blomquist G., Malmberg P. Collection of airborne micro-organisms on Nuclepore filters, estimation and analysis--CAMNEA method. J Appl Bacteriol. 1986 Nov;61(5):401–406. doi: 10.1111/j.1365-2672.1986.tb04303.x. [DOI] [PubMed] [Google Scholar]
- Peto S., Powell E. O. The assessment of aerosol concentration by means of the Andersen sampler. J Appl Bacteriol. 1970 Sep;33(3):582–598. doi: 10.1111/j.1365-2672.1970.tb02237.x. [DOI] [PubMed] [Google Scholar]
- SHIPE E. L., TYLER M. E., CHAPMAN D. N. Bacterial aerosol samplers. II. Development and evaluation of the Shipe sampler. Appl Microbiol. 1959 Nov;7:349–354. doi: 10.1128/am.7.6.349-354.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid T., Schokkin E., Boleij J. S., Heederik D. Enumeration of viable fungi in occupational environments: a comparison of samplers and media. Am Ind Hyg Assoc J. 1989 May;50(5):235–239. doi: 10.1080/15298668991374570. [DOI] [PubMed] [Google Scholar]
- Solomon W. R. Assessing fungus prevalence in domestic interiors. J Allergy Clin Immunol. 1975 Sep;56(3):235–242. doi: 10.1016/0091-6749(75)90095-0. [DOI] [PubMed] [Google Scholar]
- TYLER M. E., SHIPE E. L. Bacterial aerosol samplers. I. Development and evaluation of the all-glass impinger. Appl Microbiol. 1959 Nov;7:337–349. doi: 10.1128/am.7.6.337-349.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYLER M. E., SHIPE E. L., PAINTER R. B. Bacterial aerosol samplers. III. Comparison of biological and physical effects in liquid impinger samplers. Appl Microbiol. 1959 Nov;7:355–362. doi: 10.1128/am.7.6.355-362.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLOCHOW H. The membrane filter technique for estimating numbers of viable bacteria: some observed limitations with certain species. Appl Microbiol. 1958 May;6(3):201–206. doi: 10.1128/am.6.3.201-206.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. V., Marthi B., Fieland V. P., Ganio L. M. Effect of aerosolization on subsequent bacterial survival. Appl Environ Microbiol. 1990 Nov;56(11):3468–3472. doi: 10.1128/aem.56.11.3468-3472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman N. J., Reist P. C., Turner A. G. Comparison of two biological aerosol sampling methods. Appl Environ Microbiol. 1987 Jan;53(1):99–104. doi: 10.1128/aem.53.1.99-104.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


