Abstract
The Y2 subtype of neuropeptide tyrosine (NPY) receptors (Y2R) and some neuropeptides have been studied with in situ hybridization in sensory and autonomic neurons of rat and monkey. Between 10% and 20% of the lumbar dorsal root ganglion (DRG) neuron profiles (NPs) contain Y2R mRNA in the rat and monkey. In rat DRGs Y2R mRNA is expressed in calcitonin gene-related peptide (CGRP)-positive, medium-sized, and large neurons, that is in a complementary fashion to the Y1R that is located in small CGRP neurons. In monkey DRGs Y2R mRNA is expressed mainly in small neurons. Peripheral axotomy up-regulates the Y2R in small and large DRG neurons in both species. Y2R and NPY mRNAs are colocalized in many large neurons in axotomized rat DRGs. Y2R mRNA is expressed in 50% of the NPs in the nodose ganglion with a modest increase after axotomy. Y2R mRNA is detected in a few NPs in normal rat superior cervical ganglia, with a marked increase after transection of the carotid nerves. No Y2R mRNA-positive, but many (≈30%) weakly Y1R mRNA-positive NPs were found in the sphenopalatine ganglion. Finally, Y2R mRNA levels increase in rat spinal motoneurons after axotomy. Thus, under normal circumstances NPY may act on Y1 and Y2Rs expressed, respectively, in small and large CGRP-positive DRG neurons in the rat. Y2R may be an important receptor in the viscero-sensory neurons. Y2Rs may be particularly important after axotomy serving as presynaptic and/or autoreceptors on rat DRG, superior cervical ganglion, and nodose ganglion neurons and as presynaptic receptors in monkey DRG neurons.
Keywords: dorsal root ganglion, monkey, nerve injury, rat, sympathetic ganglia
Neuropeptide tyrosine (NPY), isolated from bovine brain by Tatemoto et al. (1), has a wide distribution in the nervous system and seems to be involved in multiple peripheral and central functions (2, 3). Based on pharmacological and binding studies using peptide fragments, evidence for the existence of multiple NPY receptor subtypes has been presented (4, 5). Two major subtypes were early recognized, the Y1 and the Y2 receptors (Y1Rs, Y2Rs) that were considered to represent primarily postjunctional and prejunctional receptors, respectively (6, 7). Y1R (8–10) and Y2R (11–13) have been cloned, and there may exist five further NPY receptor subtypes (5).
There is evidence that NPY may play a role at the spinal level. Thus, NPY-positive local neurons are present in the dorsal horn (14), but in dorsal root ganglion (DRG) neurons NPY is under normal circumstances undetectable, or present at very low levels (15). A dramatic up-regulation of this peptide occurs in DRG neurons after peripheral nerve injury, primarily in large neurons (16). Autoradiographic ligand binding studies have demonstrated strong NPY binding in the dorsal horn of the spinal cord (17, 18), which is reduced after dorsal rhizotomy (17), suggesting the presence of binding sites on DRG neurons. In fact, high affinity 125I-labeled NPY binding sites have been demonstrated on fibers of cultured DRG neurons (19). Also visceral primary afferent neurons in the rabbit nodose ganglia (NGs) express Y1 and Y2 ligand binding (20). Y2R binding on sensory neurons is increased after peripheral axotomy (Axo) (18). Using in situ hybridization, Y1R mRNA was found in a population of mainly small DRG neurons also containing calcitonin gene-related peptide (CGRP) and substance P (SP) (21), but this receptor does not appear to be transported centrifugally, thus representing a purely somatic receptor (22). Moreover, after peripheral Axo, Y1R mRNA levels decreased in small but increased in large DRG neurons (21). In the autonomic nervous system NPY coexists with noradrenaline in sympathetic neurons (23) and with acetylcholine in parasympathetic neurons (24).
In the present study we have used in situ hybridization to analyze the expression and regulation of Y2R mRNA both in rat and monkey DRGs, and in rat superior cervical ganglia (SCGs), NGs, and sphenopalatine ganglia (SPGs) under normal conditions and after Axo (DRGs in rat and monkey, and SCGs and NGs in rat).
MATERIALS AND METHODS
Tissue Preparation.
Fifteen adult male Sprague–Dawley rats (body weight, 200–250 g) and 3 male monkeys (Macaca mulatta) (3–4 kg) were deeply anesthetized, and the left sciatic nerve was transected above the trochanter major. The rats were allowed to survive for 2 (3 rats), 7 (3 rats), 14 (6 rats), or 21 (3 rats) days and monkeys for 14 days. Eighteen rats were deeply anesthetized, and the left internal and external carotid nerves were transected 2 mm away from the SCGs. The rats were sacrificed after 2, 7, or 14 days (six in each group). In another nine rats the left vagus nerve was transected 20 mm below the NG. The rats were sacrificed after 2, 7, or 14 days (three in each group). The experiments on rats had been approved by Stockholms norra djurförsöksetiska nämnd (a local ethical committee), and on monkeys by the Chinese National Committee to the Use of Experimental Animals for Medical Purposes, Shaanxi branch.
Operated animals, as well as control animals, were deeply anesthetized and the lumbar (L) spinal cord (L4–L5), L4 and L5 DRGs, SCGs, NGs, and SPGs were dissected out, frozen and cut at 5 or 14 μm in a cryostat and processed for in situ hybridization (21). Nine rats with carotid nerve cuts (as above) and three controls were anesthetized and perfusion fixed with formalin. The SCGs were postfixed, cut at 14 μm in a cryostat, and processed for immunohistochemistry (15).
Preparation of Probes and Hybridization Procedure.
A mixture of three commercially acquired oligonucleotide probes (Scandinavian Gene Synthesis, Köping, Sweden) were used: (i) TGCTTGGAGATCTTGCTCTCCAGGTGGTAGACAATGCAAC, (ii) TGTGCCTTCGCTGATGGTAATGGTCACTTGCAGCTCCAGGAC, and (iii) GAGTTTGTACTCCTTCAGGTCCAGGACATGGCTGTCGA complementary to nucleotide sequences of the intracellular loops 2 and 3, and extracellular loop 3 of the rat Y2R, respectively (25). The probe for NPY Y1R mRNA was complementary to nt 546–585 of rat NPY Y1R (10), and for NPY Y5R mRNA to nt 1542–1580 of the rat Y5R (26). Sequences for neuropeptide probes were complementary to nt 1671–1714 of rat NPY (27), nt 664–698 of rat CGRP (28), and nt 145–192 of rat SP (29). The oligonucleotide probes were labeled at the 3′ end with [α-35S]dATP (New England Nuclear) and purified as described (21). DTT was added to a final concentration of 10 mM (specific activities 1–4 × 106 cpm/ng per oligonucleotide). The in situ hybridization procedure followed published protocols (21). After hybridization the sections were rinsed, dehydrated, dried, dipped in NTB2 nuclear track emulsion (Kodak), exposed at 4°C for 2–4 weeks, developed, fixed, rinsed, and analyzed in a Nikon Microphot-FX microscope equipped with a dark-field condenser or stained with cresyl violet and mounted for viewing under bright-field. Control hybridizations with an excess of cold probe (100-fold) abolished all signals.
Immunohistochemistry.
The sections were incubated with NPY antiserum [1:800; J. Walsh, Center for Ulcer Research and Education (CURE), Los Angeles], processed for the ABC method (Vector Laboratories), and examined described (15). For control, the NPY antisera were preabsorbed with synthetic NPY (Peninsula Laboratories) at 10−6 M, which completely blocked the staining.
Quantification.
The percentage of labeled neuron profiles (NPs) were counted on cresyl violet stained sections in the Nikon microscope (21). Every fourth section of a series (in total 5–8 sections of each L5 DRG), or every third section of a series (in total 5–7 sections from each SCG or NG), or every fourth section of a series (in total 10 sections of each spinal cord) from three axotomized animals and three injured animals at each time point were selected. A total of 912-1297 NPs from each DRG of the rats, 1281–1742 NPs from each DRG of the monkeys, 958-1414 NPs from each rat SCG or NG, and 74–123 rat motoneuron profiles from both spinal ventral horns were analyzed. The number of labeled NPs was divided by the total number of NPs.
The size of NPs in the DRGs, the intensity of labeling of the Y2R mRNA in DRGs, and the intensity of labeling of NPY mRNA in SCGs were measured as described (21) using National Institutes of Health image software (courtesy W. Rasband, National Institute of Mental Health). The size of 200 NPs containing Y2R mRNA and 400 NPs with or without Y2R mRNA from both control and ipsilateral DRGs of the rats and the monkeys 14 days after unilateral Axo were measured. The relative density levels of labeling of Y2R mRNA in DRGs of three control and three axotomized (14 days) animals, and of NPY mRNAs in SCGs of three control and three axotomized (7 days) rats were measured. Fifty NPs from each DRG or SCG of each animal were measured. Each image was digitized with 256 gray levels for each picture element. The data were assessed with the unpaired two-tailed t test.
RESULTS
Rat and Monkey DRGs.
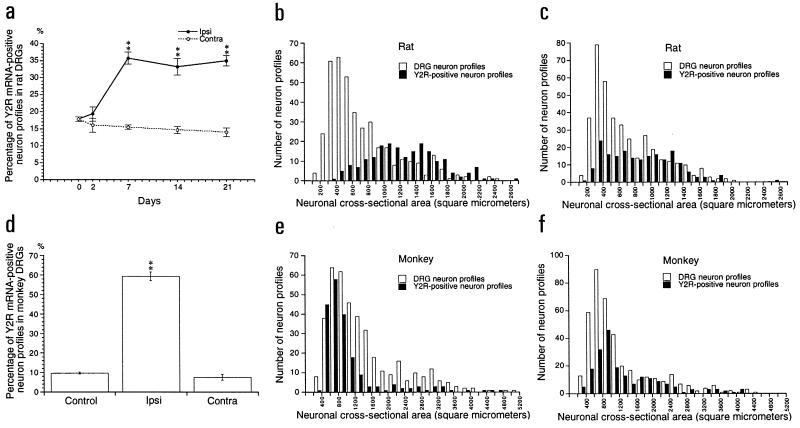
In normal rat L4 and L5 DRGs, 15–20% of all NPs were Y2R mRNA-positive (mRNA+) (Figs. 1 a and e, and 2a), with a range of 400-2700 μm2 (majority 900-1800 μm2) (Fig. 2b), that is mainly representing large neurons. After Axo there was a strong increase in the percentage of Y2R mRNA+ NPs in ipsilateral DRGs (Figs. 1 b and f, and 2a). At 7, 14, and 21 days after Axo ≈35% of all NPs were positive, with a small decrease on the contralateral side (Fig. 2a). The size of the Y2R mRNA+ NPs after Axo ranged between 300 and 1500 μm2, which is a shift toward smaller neurons (Fig. 2c). Also the intensity of the mRNA signal increased after Axo (mean of gray level of labeling ± SEM: 15.0 ± 1.5 in control rats versus 24.2 ± 1.7 14 days after Axo; n = 3, P < 0.05).
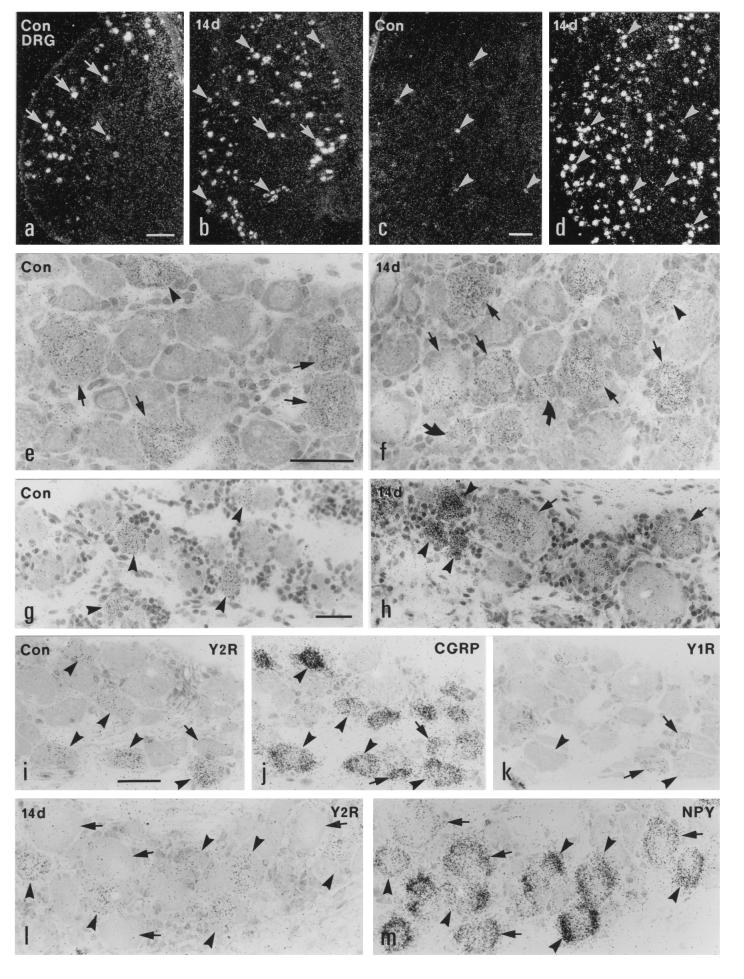
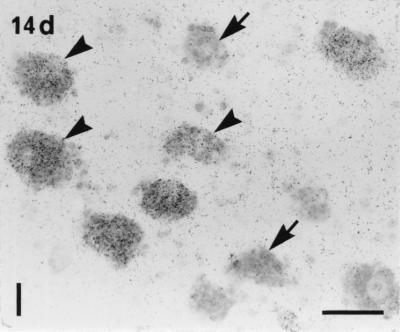
Figure 1.
Dark- (a–d) and bright- (e–m) field micrographs of control (a, c, e, g, and i–k) and ipsilateral (b, d, f, h, l, and m) L5 DRGs of the rat (a, b, e, f, and i–m) and the monkey (c, d, g, and h), hybridized with probes for Y2R (a–i and l), CGRP (j), Y1R (k), and NPY (m) mRNAs, 14 days after unilateral sciatic nerve cut. (a–h) Strong labeling of Y2R mRNA is observed in many large DRG neurons (arrows in a and e) and some medium-sized DRG neurons (arrowheads in a and e) in the normal rat DRGs. After Axo, the number of Y2R mRNA+ neurons is increased in the ipsilateral DRGs of the rat (b). More small (curved arrows), medium-sized (arrowheads), and large (arrows) neurons express Y2R mRNA (b and f). In normal monkey DRGs only a few small neurons express Y2R mRNA (arrowheads in c and g). The number of Y2R mRNA+ small (arrowheads in d and h) and large (arrows in h) neurons is markedly increased in the ipsilateral DRGs of the monkey 14 days after Axo. (i–k) Three 5-μm-thick adjacent sections of a normal rat DRG show that Y2R is expressed in CGRP mRNA+ medium-sized and large (arrowheads) neurons; however, the small neurons expressing Y1R and CGRP do not have Y2R (arrows). (l and m) Two adjacent sections of a ipsilateral rat DRG 14 days after Axo show colocalization of Y2R and NPY mRNAs in many large and medium-sized DRG neurons (arrowheads). There are some NPY mRNA+ large neurons lacking Y1R mRNA (arrows in l and m). (Bars = 200 μm in a–d and 50 μm in e–m.)
Figure 2.
Percentage of Y2R mRNA+ NPs in control, ipsilateral, and contralateral DRGs of the rats 2, 7, 14, and 21 days (a) after and of monkeys 14 days after sciatic nerve cut (d). Size range of Y2R mRNA+ NPs in rat (b) and monkey (e) control DRGs and in rat (c) and monkey (f) DRGs 14 days after Axo. The data are presented as mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.01 as compared with the control.
In normal monkey DRGs ≈10% of all NPs were Y2R mRNA+ (Figs. 1 c and g, and 2d), with a dramatic increase after Axo (up to 60%) (Figs. 1 d and h, and 2d). In normal DRGs the majority of Y2R mRNA+ NPs were of the small size (500–1400 μm2, peak at 700 μm2) largely overlapping with the distribution of all NPs (Fig. 2e). After Axo there was a shift toward larger Y2R mRNA+ NPs (Fig. 2f). Only single Y1R mRNA+ NPs could be observed in normal and axotomized DRGs (data not shown).
In 5-μm-thick adjacent sections of normal DRGs, Y2R mRNA was observed in CGRP mRNA+ medium-sized and large NPs (Fig. 1 i and j) (34.2% of the NPs contained Y2R plus CGRP mRNA, 0% only Y2R mRNA, and 65.8% only CGRP mRNA; n = 82). Fewer NPs expressed Y2R plus SP mRNA than Y2R plus CGRP mRNA (12.6% contained both Y2R plus SP mRNA; 21.4% only Y2R mRNA; 66% only SP mRNA; n = 103). Colocalization of Y2R and Y1R mRNAs was not observed (n = 60) (Fig. 1 i, j, and k). In ipsilateral DRGs 14 days after Axo, many large NPs expressed NPY mRNA and 41.8% of counted NPY mRNA+ NPs contained Y2R mRNA (Fig. 1 i and m) (35.0% contained Y2R plus NPY mRNA; 16.3% only Y2R mRNA; 48.7% only NPY mRNA; n = 80). Occasionally, Y2R and Y1R mRNA could be observed in the same NPs (2.4% contained Y2R plus Y1R mRNA; 55.4% only Y2R mRNA; 42.2% only Y1R mRNA; n = 83).
Rat Autonomic Ganglia.
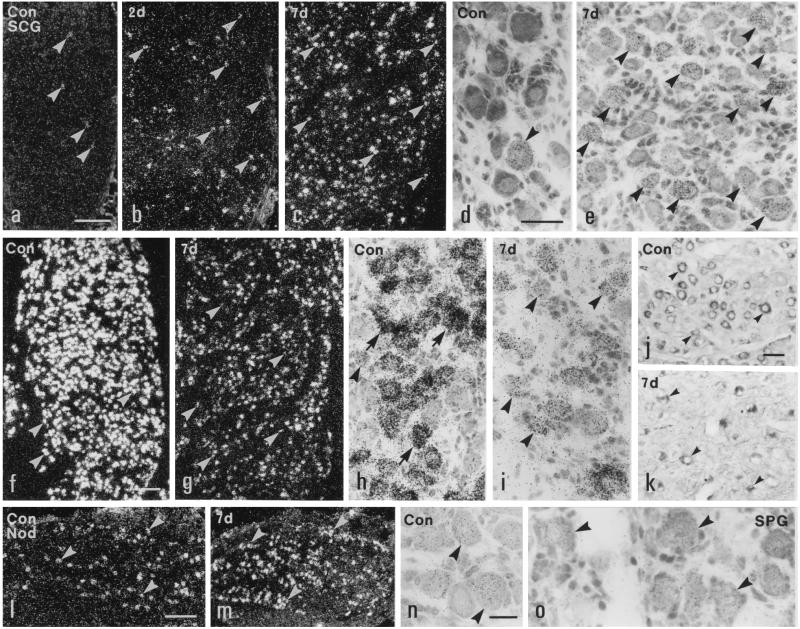
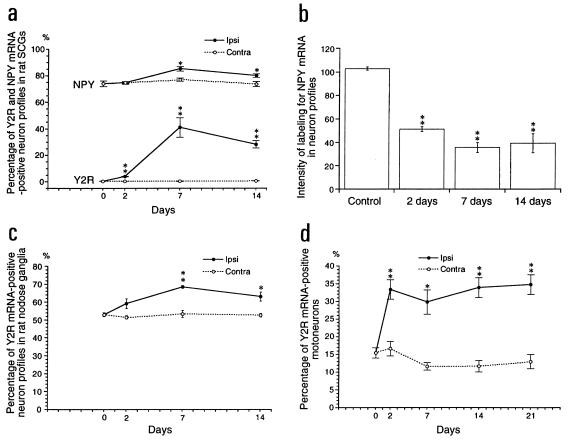
Y2R mRNA was expressed in 0.5% of the NPs in normal SCGs (Figs. 3 a and d, and 4a). Two days after transection of internal and external carotid nerves, the number of Y2R mRNA+ NPs was markedly increased ipsilaterally (Figs. 3b and 4a) and reached 40% of all NPs 7 days after Axo (Figs. 3 c and e, and 4a). No detectable change was found in contralateral SCGs (Fig. 4a). Y1R mRNA was not detected in SCGs of normal rats and/or after carotid nerve cut (data not shown).
Figure 3.
Dark- (a–c, f, g, l, and m) and bright- (d, e, h–k, n, and o) field micrographs of control (a, d, f, h, j, l, n, and o) and ipsilateral (b, c, e, g, i, k, and m) SCGs (a–k), NGs (l–n), and SPGs (o) of rats, hybridized with probes for Y2R (a–e and l–n), Y1R (o), and NPY (f–i) mRNAs, or immunolabeled for NPY (j and k) 2 (b) or 7 (c, e, g, i, k, and m) days after unilateral transection of both carotid nerves. Few neurons contain Y2R mRNA in normal SCG (arrowheads in a and d) with a small increase 2 days (arrowheads in b), and a marked increase 7 days after Axo (arrowheads in c and e). Most neurons in normal SCGs contain NPY mRNA (arrowheads in f and h) and NPY-like immunoreactivity (arrowheads in j). The intensity of labeling of NPY mRNA is decreased in the neurons (arrowheads in g and i) in ipsilateral SCG 7 days after Axo. The number of NPY+ neurons in ipsilateral SCG is markedly reduced (arrowheads in k). Many neurons in a normal NG express Y2R mRNA (arrowheads in l and n). More NG neurons are Y2R mRNA+ 7 days after Axo (arrowheads in m). Y1R mRNA is expressed in SPG neurons (arrowheads in o). (Bars = 200 μm in a–c, f, g, l, and m; 50 μm in d, e, and h–l; and 20 μm in n and o.)
Figure 4.
Percentage of Y2R mRNA+ and NPY mRNA+ NPs (a), and gray levels of NPY mRNA labeling (b) in SCGs of control rats and in ipsilateral and contralateral SCGs 2, 7, and 14 after unilateral carotid nerve cut. Percentage of Y2R mRNA+ NPs in NGs (c) and in spinal ventral horns (d) of ipsilateral and contralateral side. The data are presented as mean + SEM. ∗, P < 0.05; ∗∗, P < 0.01 as compared with the control.
In normal SCGs 74% of the counted NPs contained NPY mRNA (Figs. 3 f and h, and 4a), with a significant increase (to 85%) 7 days after Axo (Figs. 3 g and i, and 4a). However, the intensity of labeling was markedly reduced (Figs. 3 g and i, and 4b). This was paralleled by a decrease in NPY-like immunoreactivity, resulting in a clear decrease in number of positive NPs in ipsilateral SCGs (Fig. 3 j and k).
In normal NGs about 50% of all NPs expressed Y2 mRNA (Fig. 3 l and n) and a modest increase was observed 7 (Fig. 3m) and 14 days after Axo (Fig. 4c). In the SPG 32.3% of the NPs were Y1 mRNA+ (Fig. 3o), but Y2 mRNA could not be detected (data not shown).
Rat Spinal Motoneurons.
Y2R mRNA was detected in 15% of counted motoneuron profiles in the normal spinal cord (Fig. 4d). After sciatic cut a marked increase in the number of Y2R mRNA+ motoneuron profiles was seen ipsilaterally at all time intervals studied (Figs. 4d and 5).
Figure 5.
Bright-field micrograph of the ventral horn of rat spinal cord 14 days after Axo shows Y2R mRNA+ motoneurons (arrowheads). Arrows point to Y2R mRNA-negative motoneurons. (Bar = 50 μm.)
Y5 mRNA.
Y5 mRNA was not detected in L5 DRGs, SCGs, SPs, NGs, or spinal cord of rat under normal circumstances or after Axo (DRGs, SCGs, and NGs; data not shown). However, this probe showed a signal in several brain areas, including hippocampus.
DISCUSSION
The present results demonstrate that a subpopulation of mainly large neurons in rat DRGs, constituting about 15–20% of all NPs, express detectable Y2R mRNA levels under normal circumstances in agreement with a preliminary report by Gustafsson et al. (30). The corresponding figure for the NG is around 50%. In the SCG, a primarily noradrenergic sympathetic ganglion, the Y2R levels are under normal circumstance very low. No Y2R mRNA signal is detected in the normal, parasympathetic SPG. In the monkey 10% of the DRG NPs are Y2R mRNA+, but the vast majority is small. In several ganglia Axo causes a marked up-regulation of Y2R mRNA levels with doubling in the rat DRG NPs, a 6-fold increase in the monkey DRG NPs, and in the rat SCGs around 40% of the NPs now express the receptor (up from 0%). Also Y2 mRNA in rat motoneurons increases (from 15 to 35% of the NPs). The increase in NGs was, however, modest. Moreover, in all cases mRNA levels in individual neurons are elevated, suggesting increased receptor protein synthesis and levels. It should be emphasized that our methodology may not be sensitive enough to detect low levels of Y2R mRNA, and that we therefore underestimate the occurrence of Y2Rs. The present results, together with previously published data, have been summarized in Table 1.
Table 1.
NPY receptors in sensory and autonomic ganglia
| Y2R mRNA
|
Y1R mRNA*
|
Y1R protein†
|
Binding‡
|
|||||
|---|---|---|---|---|---|---|---|---|
| Normal | Axo | Normal | Axo | Normal | Axo | Normal | Axo | |
| Rat | ||||||||
| DRGs | ||||||||
| Small neurons | 0 | + | +++ | + | +++ | 0 | +++ | + |
| Large neurons | ++ | +++ | 0 | ++ | 0 | ? | + | +++ |
| Processes (DRGs) | ND | ND | ND | ND | ND | ND | + | +++ |
| Dorsal roots | ND | ND | ND | ND | ND | ND | (+) | ++ |
| Dorsal horn | ||||||||
| Laminae I + II | + | + | +++ | +++ | +++ | +++ | +++ | ++++ |
| Laminae III + IV | + | + | 0 | 0 | 0 | 0 | + | +++ |
| NG | +++ | ++++ | 0 | 0 | 0 | 0 | ND | ND |
| SCG | (+) | +++ | 0 | 0 | 0 | 0 | ND | ND |
| SPG | 0 | ND | ++§ | ND | ND | ND | ND | ND |
| Monkey | ||||||||
| DRG | ++ | ++++ | (+) | (+) | ND | ND | ? | ? |
| DH | + | + | 0 | 0 | 0 | 0 | ++++ | +++ |
Sensory Ganglia.
In a previous study on the rat, peripheral Axo caused a marked increase in 125I-labeled peptide tyrosine tyrosine (PYY) binding over dorsal roots, and over both the superficial and deep layers of the dorsal horn (18). Competition experiments indicated that this binding mainly represents a Y2R. These findings and the fact that small and large neurons project, respectively, to the superficial and deeper laminae of the dorsal horn agree with the present results, showing that Y2 mRNA after Axo is up-regulated both in small and large neurons.
The present and previous results show that CGRP neurons can be subdivided into two complementary subpopulations, small CGRP neuron expressing the Y1R (21) and large CGRP neurons expressing Y2 mRNA (present data). After Axo we frequently found Y2R mRNA in large neurons also containing NPY mRNA. Also the Y1R is up-regulated in large neurons after nerve injury (21). A distinct difference between the two receptors is, however, that the Y1Rs remain in the cell body as somatic receptors (22), whereas the Y2Rs are transported centrally to the dorsal horn, in high amounts especially after Axo (18), in agreement with their postulated roles as post- and presynaptic receptors, respectively (6). Thus, after Axo, Y2Rs may act as autoreceptors on primary afferents in the dorsal horn, in addition to being activated by NPY released from local dorsal horn neurons (14). NPY has been reported to inhibit Ca2+ influx into cultured rat DRG neurons via Y2Rs (31, 32) and to inhibit release of SP from the terminals both in vitro and in vivo (19, 33). Spinally administered NPY induces antinociceptive effects (34) and biphasic effects on the nociceptive flexor reflex (35). The inhibitory effects of NPY may perhaps be enhanced after Axo, because more receptors should be available on sensory neurons.
Some species differences between rats and monkeys were observed in the present study. The Y1R is hardly synthesized normally in monkey DRGs or after Axo. However, the Y2R is expressed in small DRG neurons that often contain CGRP and SP in normal monkeys (36). After Axo up-regulation of Y2R occurs both in small and large neurons and this is more pronounced in monkey than in rat. This is in agreement with the results of a receptor binding study showing that Y2Rs are the main NPY binding sites in the superficial dorsal horn of the spinal cord of normal monkeys and after Axo (18). NPY has not been detected in normal monkey DRGs, and only 2% of neurons express NPY after Axo (36). Thus, in the monkey local neurons in the superficial dorsal horn may therefore be the main source of NPY acting on the primary afferent terminals containing Y2Rs.
Autonomic Ganglia.
The present study indicates that the Y2R normally is expressed only at very low levels in SCGs, suggesting that this presynaptic autoreceptor (6) is only of small functional significance, and that NPY released from sympathetic nerve endings may mainly act on postsynaptic receptors. Interestingly, no Y2R mRNA signal was observed in SPGs, which instead showed a low expression of Y1R mRNA in many neurons.
A marked increase in Y2R mRNA was observed in sympathetic ganglia after Axo close to the cell bodies, in parallel with reduced NPY levels. Also, although more neurons produced NPY peptide, the levels in individual neurons decreased, as also reported by others (37). The functional significance of an increase in “prejunctional” receptors or sympathetic neurons after Axo is unclear. NPY has been shown to contribute to peripheral hyperalgesia by acting on prejunctional Y2Rs on postganglionic sympathetic terminals (38), which could be present at high levels on sympathetic fibers regenerating after Axo.
In conclusion, the present data support the view that Y2R-mediated effects may be strengthened after nerve injury. This may offer novel approaches to treatment of, for example, pain via agents acting on Y2Rs.
Acknowledgments
We thank Drs. J. Walsh and H. Wong (Center for Ulcer Research and Education/University of California, Los Angeles; supported by National Institutes of Health Grant DK 17294) for generous donation of NPY antiserum. This work is supported by the Swedish Medical Research Council (04X-2887), Marianne and Marcus Wallenbergs Stiftelse, Gustav V:s and Drottning Victorias Stiftelse, Astra Pain Control AB, and the Nature Science Foundation of China (Grants 39525010 and 39500045).
Footnotes
Abbreviations: Axo, axotomy; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; L, lumbar; mRNA+, mRNA-positive; NP, neuron profile; NPY, neuropeptide tyrosine; YIR and Y2R, NPY1 and -2 receptors; SP, substance P; NGs, nodose ganglia; SCGs, superior cervical ganglia; SPGs, sphenopalatine ganglia.
References
- 1.Tatemoto K, Carlquist M, Mutt V. Nature (London) 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 2.Allen, J. J. & Koenig, J. I., eds. (1992) Ann. N.Y. Acad. Sci. 611.
- 3.Lundberg J M. Pharmacol Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- 4.Wahlestedt C, Reis D J. Annu Rev Pharmacol Toxicol. 1993;32:309–352. doi: 10.1146/annurev.pa.33.040193.001521. [DOI] [PubMed] [Google Scholar]
- 5.Larhammar D. Regul Pept. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- 6.Wahlestedt C, Yanaihara N, Håkanson R. Regul Pept. 1986;13:307–318. doi: 10.1016/0167-0115(86)90048-0. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh S P, Håkanson R, Schwartz T W. FEBS Lett. 1989;245:209–214. doi: 10.1016/0014-5793(89)80223-6. [DOI] [PubMed] [Google Scholar]
- 8.Herzog H, Hort Y J, Ball H J, Hayes G, Shine J, Selbie L A. Proc Natl Acad Sci USA. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larhammar D, Blomqvist A G, Yee F, Jazin E, Yoo H, Wahlestedt C. J Biol Chem. 1992;267:10935–10938. [PubMed] [Google Scholar]
- 10.Eva C, Oberto A, Sprengel R, Genazzani E. FEBS Lett. 1992;314:285–288. doi: 10.1016/0014-5793(92)81490-d. [DOI] [PubMed] [Google Scholar]
- 11.Gerald C, Walker M W, Vaysse P J-J, He C, Branchek T A, Weinshank R L. J Biol Chem. 1995;270:26758–26761. doi: 10.1074/jbc.270.45.26758. [DOI] [PubMed] [Google Scholar]
- 12.Gehlert D R, Beavers L S, Johnson D, Gackenheimer S L, Schober D A, Gadski R A. Mol Pharmacol. 1996;49:224–228. [PubMed] [Google Scholar]
- 13.Rose P M, Fernandes P, Lynch J S, Fraxier S T, Fisher S M, Kodukula K, Kienzle B, Seethala R. J Biol Chem. 1995;270:22661–22664. doi: 10.1074/jbc.270.39.22661. [DOI] [PubMed] [Google Scholar]
- 14.Gibson J J, Polak J M, Allen J M, Adrian T E, Kelly J S, Bloom S R. J Comp Neurol. 1984;227:78–91. doi: 10.1002/cne.902270109. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Nicholas A P, Hökfelt T. Neuroscience. 1993;57:365–384. doi: 10.1016/0306-4522(93)90069-r. [DOI] [PubMed] [Google Scholar]
- 16.Wakisaka S, Kajander K C, Bennett G J. Neurosci Lett. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 17.Kar S, Quirion R. Brain Res. 1992;574:333–337. doi: 10.1016/0006-8993(92)90836-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Ji R-R, Nilsson S, Villar M, Ubink R, Ju G, Wiesenfeld-Hallin Z, Hökfelt T. Eur J Neurosci. 1995;7:367–380. doi: 10.1111/j.1460-9568.1995.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker M W, Ewald D A, Perney T M, Miller R G. J Neurosci. 1988;8:2438–2446. doi: 10.1523/JNEUROSCI.08-07-02438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghilardi J R, Allen C J, Vigna S R, McVey D C, Mantyh P W. Brain Res. 1994;633:33–40. doi: 10.1016/0006-8993(94)91519-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Wiesenfeld-Hallin Z, Hökfelt T. Eur J Neurosci. 1994;6:43–57. doi: 10.1111/j.1460-9568.1994.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Bao L, Xu Z Q, Jutta K, Arvidsson U, Elde R, Hökfelt T. Proc Natl Acad Sci USA. 1994;91:11738–11442. doi: 10.1073/pnas.91.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundberg J M, Terenius L, Hökfelt T, Goldstein M. Neurosci Lett. 1983;42:167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- 24.Leblanc G G, Trimmer B A, Landis S C. Proc Natl Acad Sci USA. 1987;84:3511–3515. doi: 10.1073/pnas.84.10.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerald, C., Walker, M. W., Branchek, T. & Weinsham, R. (1995) Int. Patent Application WO 95/21245.
- 26.Gerald C, Walker M W, Criscione L, Gustafson E, Batzi-Hartmann C, Smith K E, Vaysse P, Durkin M M, Laz T M, Linemeyer D L, Schaffhauser A O, Whitebread S, Hofbauer K G, Taber R I, Branchek T A, Weinshank R L. Nature (London) 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- 27.Larhammar D, Ericsson A, Persson H. Proc Natl Acad Sci USA. 1987;84:2068–2072. doi: 10.1073/pnas.84.7.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amara S G, Arriza J L, Leff S E, Swanson L W, Evans R M, Rosenfeld M G. Science. 1985;229:1094–1097. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- 29.Krause J E, Chirgwin J M, Carter M S, Xu Z S, Hershey D. Proc Natl Acad Sci USA. 1987;84:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafsson E L, Durkin M M, Smith K E, Gerald C, She Y, Illy A I, Weinshank R W, Branchek T A. Soc Neurosci Abstr. 1995;21:2048. [Google Scholar]
- 31.Bleakman D, Colmers W F, Fournier A, Miller R J. Br J Pharmacol. 1991;103:1781–1789. doi: 10.1111/j.1476-5381.1991.tb09863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewald D A, Matthies H J G, Perney T M, Walker M W, Miller R J. J Neurosci. 1988;8:2447–2451. doi: 10.1523/JNEUROSCI.08-07-02447.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duggan A W, Hope P J, Lang C W. Neuroscience. 1991;44:733–740. doi: 10.1016/0306-4522(91)90092-3. [DOI] [PubMed] [Google Scholar]
- 34.Hua X Y, Boublik J H, Spicer M A, Rivier J E, Brown M R, Yaksh T L. J Pharmacol Exp Ther. 1991;258:243–248. [PubMed] [Google Scholar]
- 35.Xu X-J, Hao J-X, Hökfelt T, Wiesenfeld-Hallin Z. Neuroscience. 1994;63:817–826. doi: 10.1016/0306-4522(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Ju G, Elde R, Hökfelt T. J Neurocytol. 1993;22:342–381. doi: 10.1007/BF01195558. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Zigmond R E. Eur J Neurosci. 1996;8:2213–2220. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 38.Tracey D J, Romm M A, Yao N N L. Brain Res. 1995;669:245–254. doi: 10.1016/0006-8993(94)01265-j. [DOI] [PubMed] [Google Scholar]