Abstract
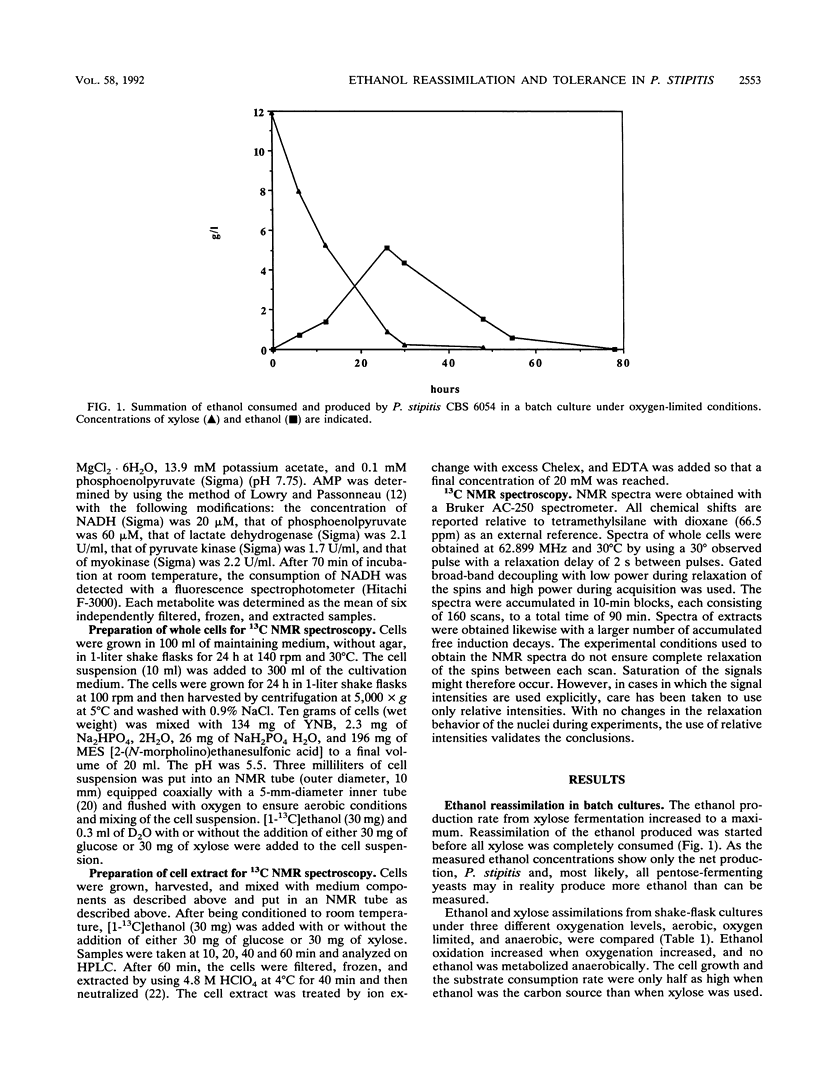
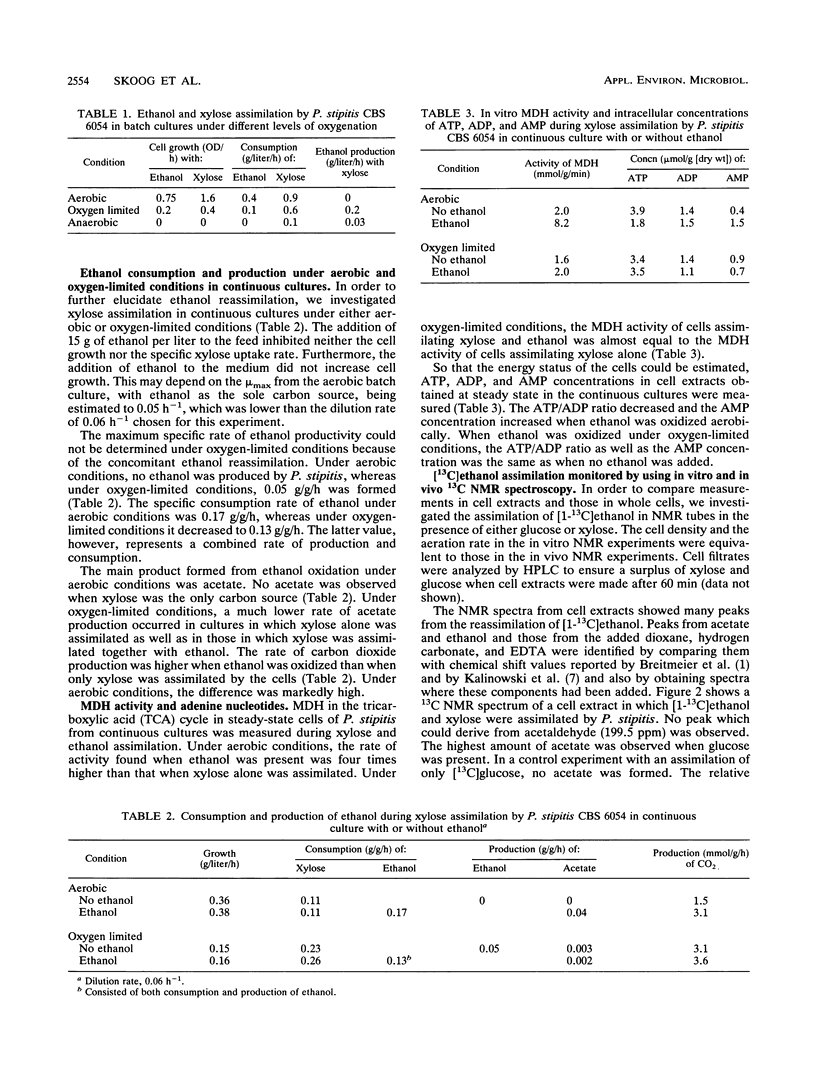
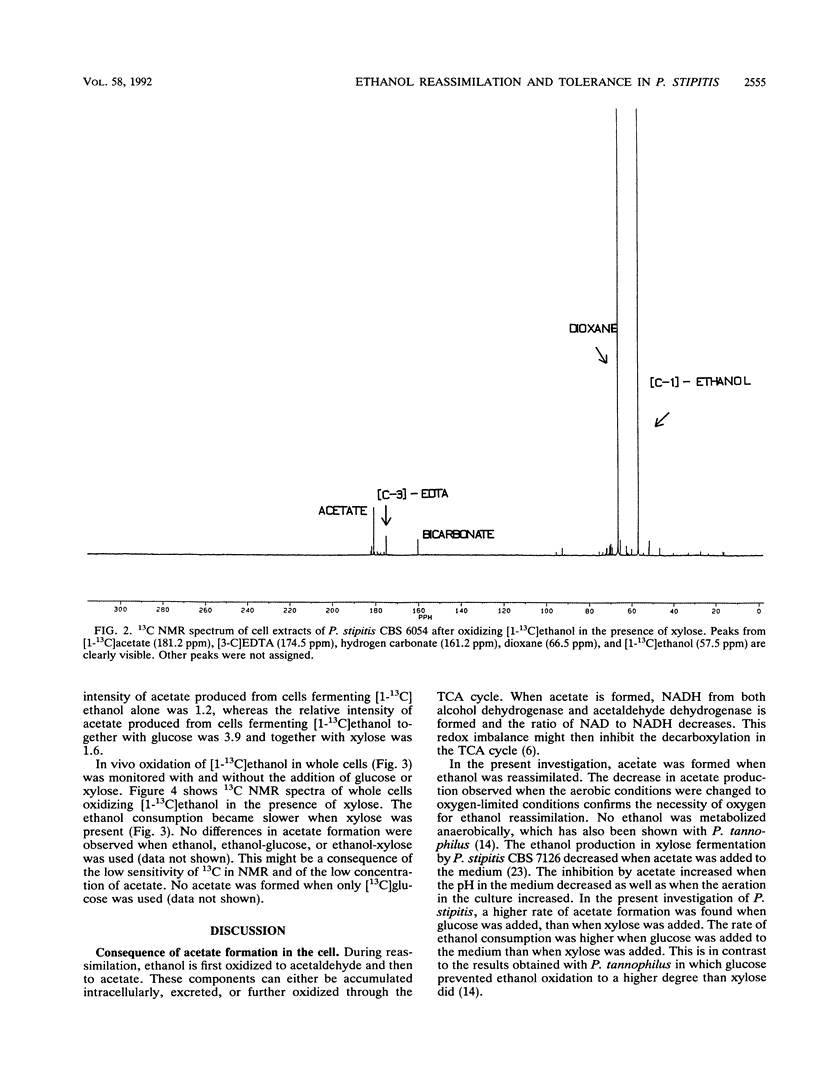
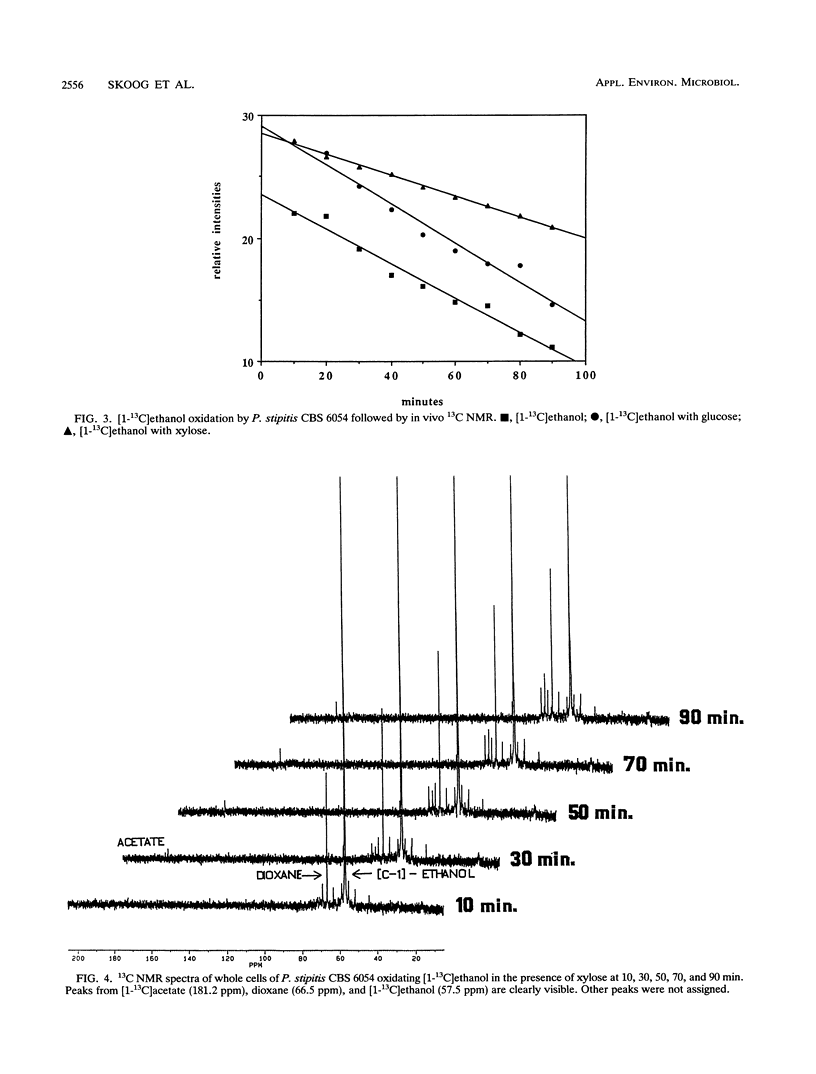
Ethanol reassimilation in Pichia stipitis CBS 6054 was studied by using continuous cultures, and the oxidation of [1-13C]ethanol was monitored by in vivo and in vitro 13C nuclear magnetic resonance spectroscopy. Acetate was formed when ethanol was reassimilated. The ATP/ADP ratio and the carbon dioxide production decreased, whereas the malate dehydrogenase activity increased, in ethanol-reassimilating cells. The results are discussed in terms of the low ethanol tolerance in P. stipitis compared with that in Saccharomyces cerevisiae (S. W. Brown, S. G. Oliver, D. E. F. Harrison, and R. C. Righelato, Eur. J. Appl. Microbiol. Biotechnol. 11:151-155, 1981).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Lieberthal S., Oldfield M., Shanley B. C. Effect of ethanol and acetaldehyde on membrane-bound enzymes in rat brain. Adv Exp Med Biol. 1980;132:797–805. doi: 10.1007/978-1-4757-1419-7_83. [DOI] [PubMed] [Google Scholar]
- Lohmeier-Vogel E., Skoog K., Vogel H., Hahn-Hägerdal B. 31P nuclear magnetic resonance study of the effect of azide on xylose fermentation by Candida tropicalis. Appl Environ Microbiol. 1989 Aug;55(8):1974–1980. doi: 10.1128/aem.55.8.1974-1980.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro-Dias M. C., Santos H. Effects of ethanol on Saccharomyces cerevisiae as monitored by in vivo 31P and 13C nuclear magnetic resonance. Arch Microbiol. 1990;153(4):384–391. doi: 10.1007/BF00249010. [DOI] [PubMed] [Google Scholar]
- Lutstorf U., Megnet R. Multiple forms of alcohol dehydrogenase in Saccharomyces cerevisiae. I. Physiological control of ADH-2 and properties of ADH-2 and ADH-4. Arch Biochem Biophys. 1968 Sep 10;126(3):933–944. doi: 10.1016/0003-9861(68)90487-6. [DOI] [PubMed] [Google Scholar]
- Maleszka R., Schneider H. Concurrent Production and Consumption of Ethanol by Cultures of Pachysolen tannophilus Growing on d-Xylose. Appl Environ Microbiol. 1982 Oct;44(4):909–912. doi: 10.1128/aem.44.4.909-912.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim D., Young E. T. Primary structure requirements for correct sorting of the yeast mitochondrial protein ADH III to the yeast mitochondrial matrix space. Mol Cell Biol. 1987 Jan;7(1):294–304. doi: 10.1128/mcb.7.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog K., Hahn-Hägerdal B. Effect of Oxygenation on Xylose Fermentation by Pichia stipitis. Appl Environ Microbiol. 1990 Nov;56(11):3389–3394. doi: 10.1128/aem.56.11.3389-3394.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


