Abstract
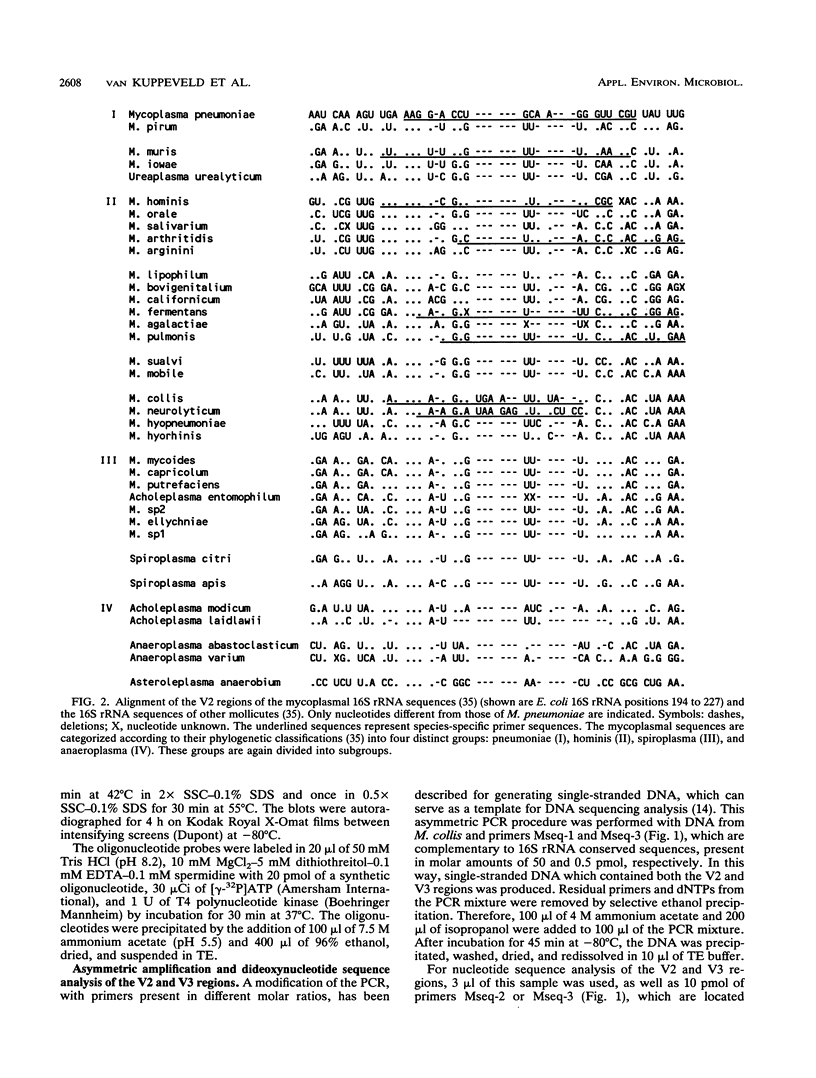
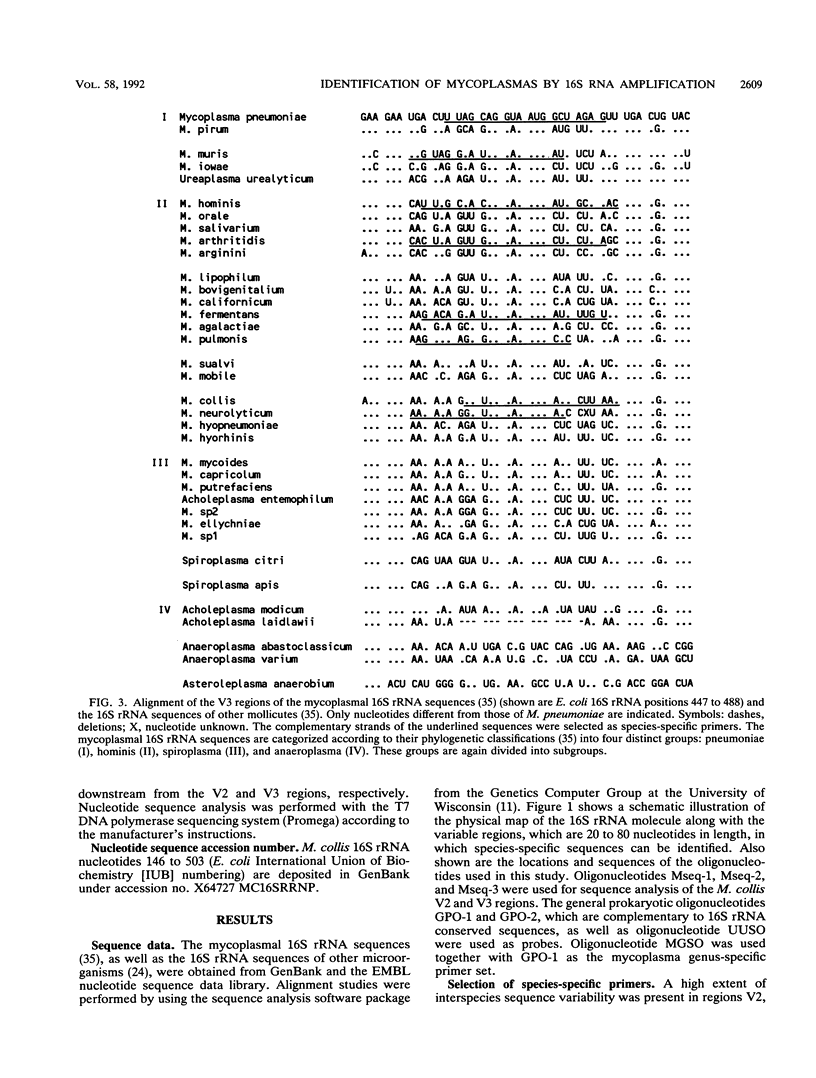
Systematic computer alignment of mycoplasmal 16S rRNA sequences allowed the identification of variable regions with both genus- and species-specific sequences. Species-specific sequences of Mycoplasma collis were elucidated by asymmetric amplification and dideoxynucleotide sequencing of variable regions, using primers complementary to conserved regions of 16S rRNA. Primers selected for Mycoplasma pneumoniae, M. hominis, M. fermentans, Ureaplasma urealyticum, M. pulmonis, M. arthritidis, M. neurolyticum, M. muris, and M. collis proved to be species specific in the polymerase chain reaction. The genus-specific primers reacted with all mycoplasmal species investigated as well as with members of the genera Ureaplasma, Spiroplasma, and Acholeplasma. No cross-reaction was observed with members of the closely related genera Streptococcus, Lactobacillus, Bacillus, and Clostridium or with any other microorganism tested. On the basis of the high copy number of rRNA, a highly sensitive polymerase chain reaction assay was developed in which the nucleic acid content equivalent to a single organism could be detected.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernet C., Garret M., de Barbeyrac B., Bebear C., Bonnet J. Detection of Mycoplasma pneumoniae by using the polymerase chain reaction. J Clin Microbiol. 1989 Nov;27(11):2492–2496. doi: 10.1128/jcm.27.11.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard A., Gautier M., Mayau V. Detection and identification of mycoplasmas by amplification of rDNA. FEMS Microbiol Lett. 1991 Jun 1;65(1):37–42. doi: 10.1016/0378-1097(91)90467-o. [DOI] [PubMed] [Google Scholar]
- Blanchard A. Ureaplasma urealyticum urease genes; use of a UGA tryptophan codon. Mol Microbiol. 1990 Apr;4(4):669–676. doi: 10.1111/j.1365-2958.1990.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Davis J. K. Respiratory and genital mycoplasmosis of laboratory rodents: implications for biomedical research. Isr J Med Sci. 1981 Jul;17(7):548–554. [PubMed] [Google Scholar]
- Claas H. C., Melchers W. J., de Bruijn I. H., de Graaf M., van Dijk W. C., Lindeman J., Quint W. G. Detection of Chlamydia trachomatis in clinical specimens by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1990 Dec;9(12):864–868. doi: 10.1007/BF01967500. [DOI] [PubMed] [Google Scholar]
- Cornelissen M. T., Smits H. L., Briët M. A., van den Tweel J. G., Struyk A. P., van der Noordaa J., ter Schegget J. Uniformity of the splicing pattern of the E6/E7 transcripts in human papillomavirus type 16-transformed human fibroblasts, human cervical premalignant lesions and carcinomas. J Gen Virol. 1990 May;71(Pt 5):1243–1246. doi: 10.1099/0022-1317-71-5-1243. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W., Sankoff D., Cedergren R. J. On the evolutionary descent of organisms and organelles: a global phylogeny based on a highly conserved structural core in small subunit ribosomal RNA. Nucleic Acids Res. 1984 Jul 25;12(14):5837–5852. doi: 10.1093/nar/12.14.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel U. B., Geiser A., Stanbridge E. J. Oligonucleotide probes complementary to variable regions of ribosomal RNA discriminate between Mycoplasma species. J Gen Microbiol. 1987 Jul;133(7):1969–1974. doi: 10.1099/00221287-133-7-1969. [DOI] [PubMed] [Google Scholar]
- Harasawa R., Koshimizu K., Takeda O., Uemori T., Asada K., Kato I. Detection of Mycoplasma hyopneumoniae DNA by the polymerase chain reaction. Mol Cell Probes. 1991 Apr;5(2):103–109. doi: 10.1016/0890-8508(91)90003-3. [DOI] [PubMed] [Google Scholar]
- Harasawa R., Koshimizu K., Uemori T., Takeda O., Asada K., Kato I. The polymerase chain reaction for Mycoplasma pulmonis. Microbiol Immunol. 1990;34(4):393–395. doi: 10.1111/j.1348-0421.1990.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Hyman H. C., Yogev D., Razin S. DNA probes for detection and identification of Mycoplasma pneumoniae and Mycoplasma genitalium. J Clin Microbiol. 1987 Apr;25(4):726–728. doi: 10.1128/jcm.25.4.726-728.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. S., Uldum S. A., Søndergård-Andersen J., Vuust J., Lind K. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol. 1991 Jan;29(1):46–50. doi: 10.1128/jcm.29.1.46-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Kwok S., Kellogg D. E., McKinney N., Spasic D., Goda L., Levenson C., Sninsky J. J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990 Feb 25;18(4):999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. C., Shih J. W., Newton P. B., 3rd, Wong D. M., Hayes M. M., Benish J. R., Wear D. J., Wang R. Y. Virus-like infectious agent (VLIA) is a novel pathogenic mycoplasma: Mycoplasma incognitus. Am J Trop Med Hyg. 1989 Nov;41(5):586–600. doi: 10.4269/ajtmh.1989.41.586. [DOI] [PubMed] [Google Scholar]
- Lo S. C., Shih J. W., Yang N. Y., Ou C. Y., Wang R. Y. A novel virus-like infectious agent in patients with AIDS. Am J Trop Med Hyg. 1989 Feb;40(2):213–226. doi: 10.4269/ajtmh.1989.40.213. [DOI] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., Hendriks L., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak-Vogelzang A. A., Reygers R., Hekkens F. E. Isolation of Mycoplasma hyorhinis and Mycoplasma fermentans from cell cultures. J Biol Stand. 1980;8(4):243–254. doi: 10.1016/s0092-1157(80)80001-1. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saillard C., Carle P., Bové J. M., Bébéar C., Lo S. C., Shih J. W., Wang R. Y., Rose D. L., Tully J. G. Genetic and serologic relatedness between Mycoplasma fermentans strains and a mycoplasma recently identified in tissues of AIDS and non-AIDS patients. Res Virol. 1990 May-Jun;141(3):385–395. doi: 10.1016/0923-2516(90)90010-g. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Furr P. M., Tully J. G. Serological cross-reactions between Mycoplasma genitalium and M. pneumoniae. Lancet. 1983 Mar 5;1(8323):527–527. doi: 10.1016/s0140-6736(83)92212-2. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Tully J. G., Furr P. M., Cole R. M., Rose D. L., Hanna N. F. Urogenital mycoplasma infections of man: a review with observations on a recently discovered mycoplasma. Isr J Med Sci. 1981 Jul;17(7):524–530. [PubMed] [Google Scholar]
- Tully J. G., Baseman J. B. Mycoplasma. Lancet. 1991 May 25;337(8752):1296–1296. doi: 10.1016/0140-6736(91)92971-4. [DOI] [PubMed] [Google Scholar]
- Waters A. P., McCuthan T. F. Ribosomal RNA: nature's own polymerase-amplified target for diagnosis. Parasitol Today. 1990 Feb;6(2):56–59. doi: 10.1016/0169-4758(90)90071-b. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]