Abstract
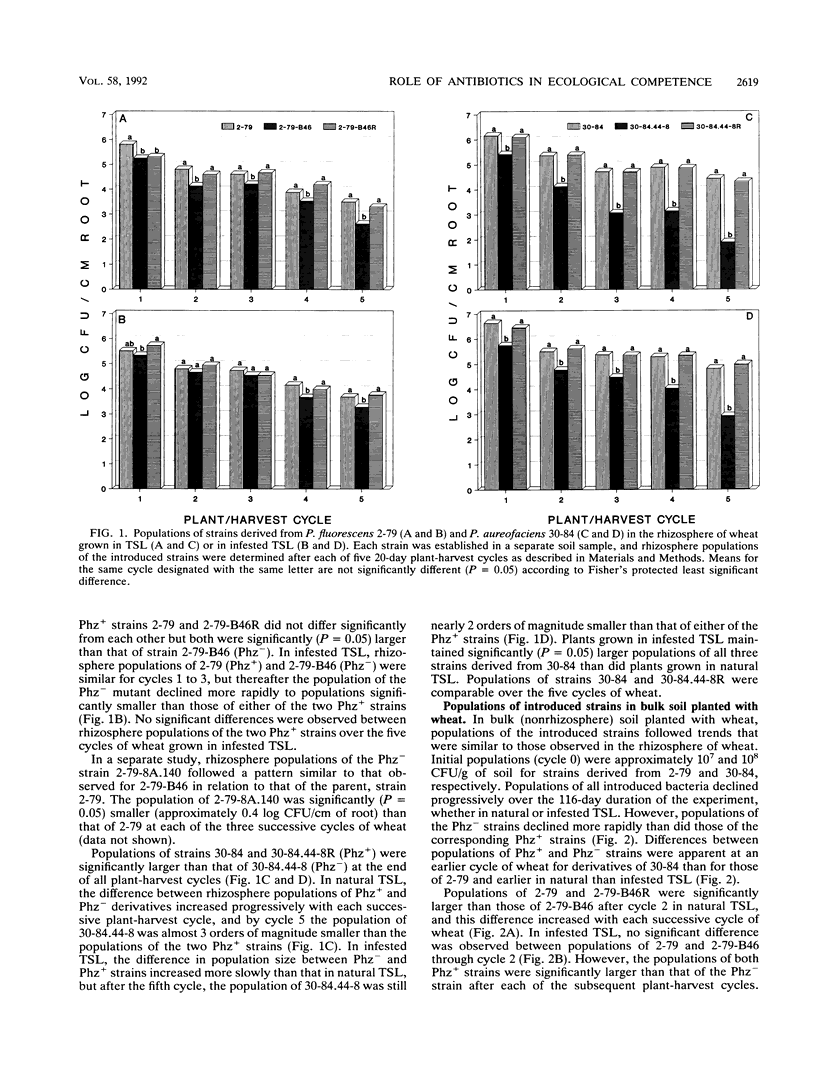
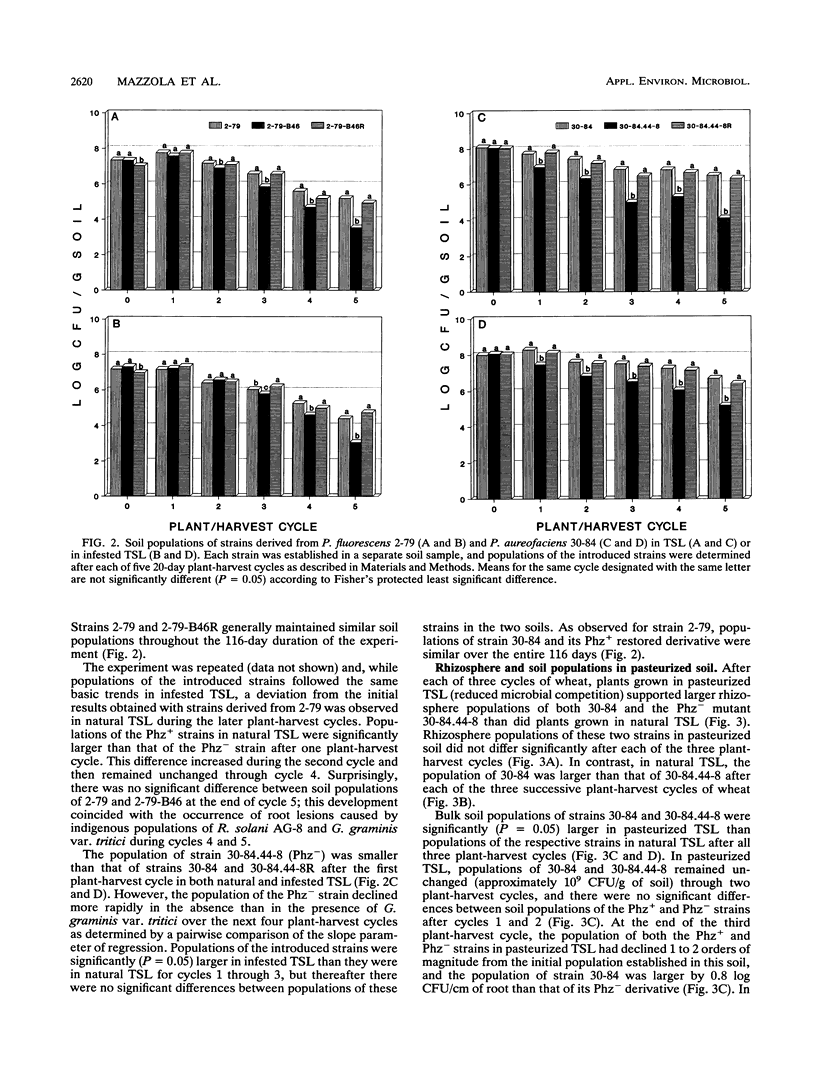
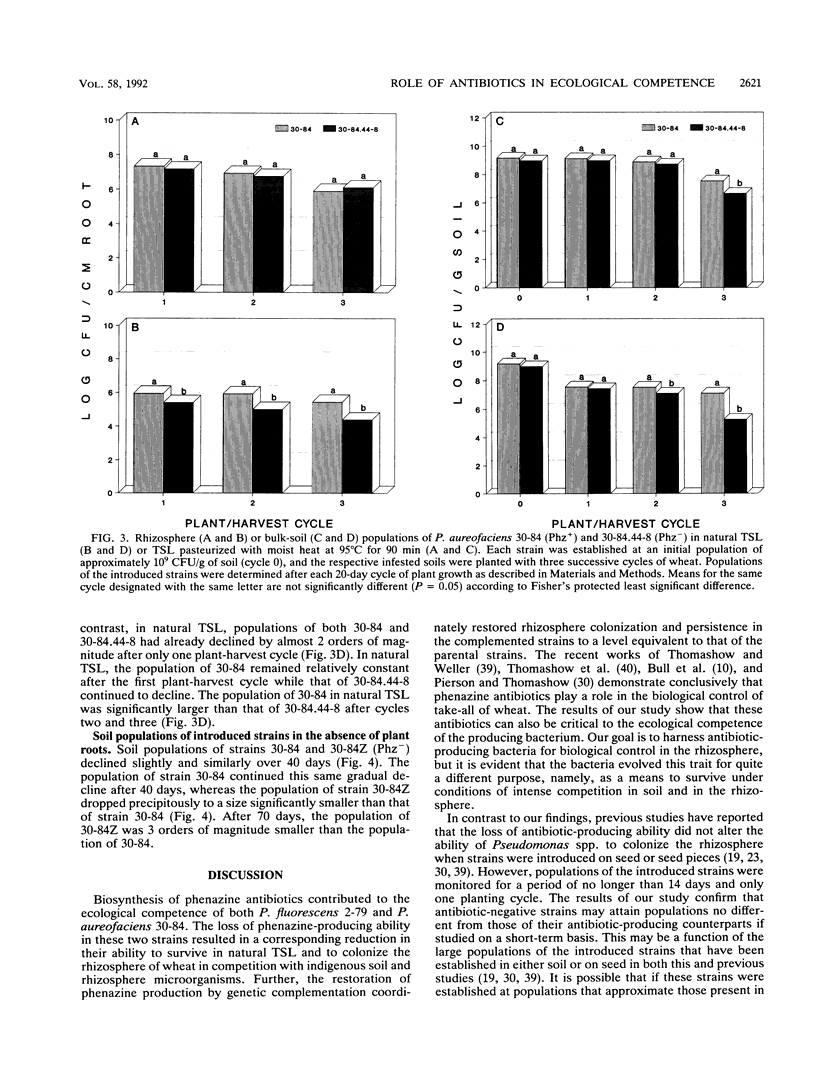
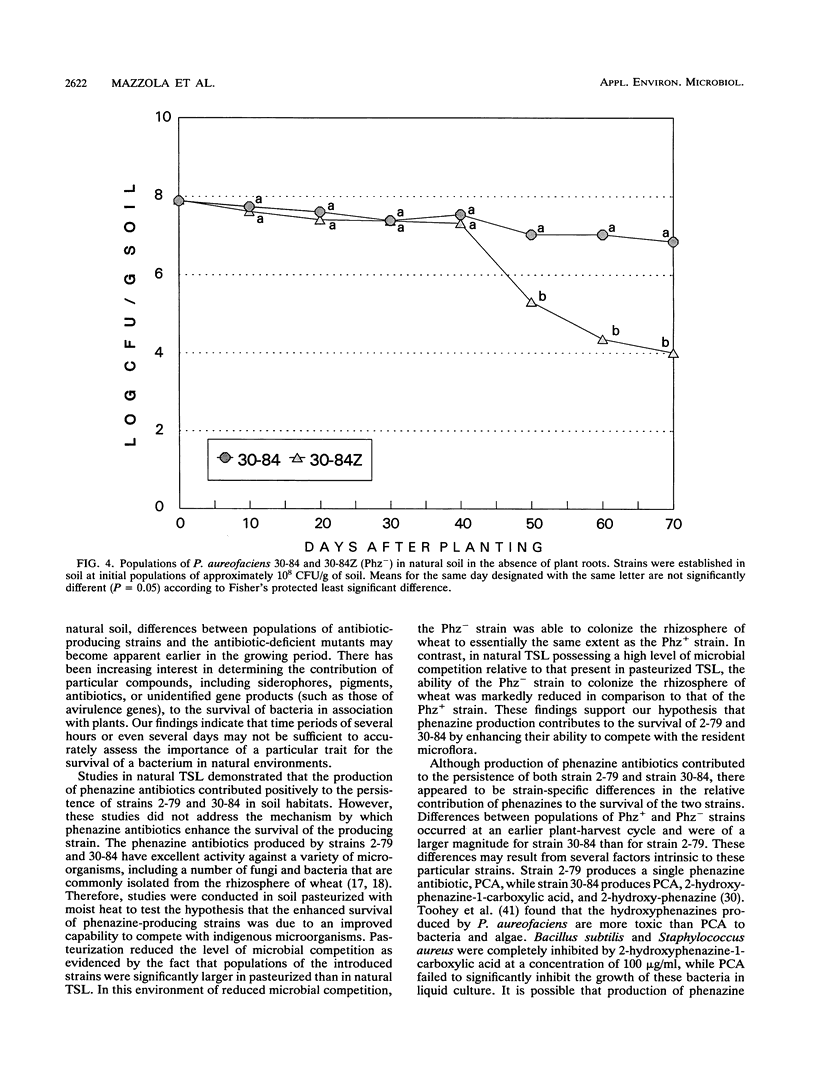
Phenazine antibiotics produced by Pseudomonas fluorescens 2-79 and Pseudomonas aureofaciens 30-84, previously shown to be the principal factors enabling these bacteria to suppress take-all of wheat caused by Gaeumannomyces graminis var. tritici, also contribute to the ecological competence of these strains in soil and in the rhizosphere of wheat. Strains 2-79 and 30-84, their Tn5 mutants defective in phenazine production (Phz-), or the mutant strains genetically restored for phenazine production (Phz+) were introduced into Thatuna silt loam (TSL) or TSL amended with G. graminis var. tritici. Soils were planted with three or five successive 20-day plant-harvest cycles of wheat. Population sizes of Phz- derivatives declined more rapidly than did population sizes of the corresponding parental or restored Phz+ strains. Antibiotic biosynthesis was particularly critical to survival of these strains during the fourth and fifth cycles of wheat in the presence of G. graminis var. tritici and during all five cycles of wheat in the absence of take-all. In pasteurized TSL, a Phz- derivative of strain 30-84 colonized the rhizosphere of wheat to the same extent that the parental strain did. The results indicate that production of phenazine antibiotics by strains 2-79 and 30-84 can contribute to the ecological competence of these strains and that the reduced survival of the Phz- strains is due to a diminished ability to compete with the resident microflora.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Habibzadegah-Tari P., Tepper C. S. Molecular Studies on the Role of a Root Surface Agglutinin in Adherence and Colonization by Pseudomonas putida. Appl Environ Microbiol. 1988 Feb;54(2):375–380. doi: 10.1128/aem.54.2.375-380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONDI A., BIRK Y. A difference in in vitro pancreatic digestion between animal and plant protein feeds. Nature. 1952 Oct 18;170(4329):673–673. doi: 10.1038/170673a0. [DOI] [PubMed] [Google Scholar]
- Better M., Helinski D. R. Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):311–316. doi: 10.1128/jb.155.1.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbane P. G., Janik L. J., Tate M. E., Warren R. F. Revised structure for the phenazine antibiotic from Pseudomonas fluorescens 2-79 (NRRL B-15132). Antimicrob Agents Chemother. 1987 Dec;31(12):1967–1971. doi: 10.1128/aac.31.12.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY H. F., HAYNES W. C., JACKSON R. W., LOCKE J. M., PRIDHAM T. G., SOHNS V. E., STODOLA F. H. Pseudomonas aureofaciens Kluyver and phenazine alpha-carboxylic acid, its characteristic pigment. J Bacteriol. 1956 Sep;72(3):412–417. doi: 10.1128/jb.72.3.412-417.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weger L. A., van der Vlugt C. I., Wijfjes A. H., Bakker P. A., Schippers B., Lugtenberg B. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol. 1987 Jun;169(6):2769–2773. doi: 10.1128/jb.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D. The production and role of antibiotics in soil. J Antibiot (Tokyo) 1976 Oct;29(10):987–1000. doi: 10.7164/antibiotics.29.987. [DOI] [PubMed] [Google Scholar]
- Gurusiddaiah S., Weller D. M., Sarkar A., Cook R. J. Characterization of an antibiotic produced by a strain of Pseudomonas fluorescens inhibitory to Gaeumannomyces graminis var. tritici and Pythium spp. Antimicrob Agents Chemother. 1986 Mar;29(3):488–495. doi: 10.1128/aac.29.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. W., Martin W. J. Microwave oven for melting laboratory media. J Clin Microbiol. 1978 Apr;7(4):401–402. doi: 10.1128/jcm.7.4.401-402.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Klein D. A., Casida L. E., Jr Escherichia coli die-out from normal soil as related to nutrient availability and the indigenous microflora. Can J Microbiol. 1967 Nov;13(11):1461–1470. doi: 10.1139/m67-194. [DOI] [PubMed] [Google Scholar]
- LEVITCH M. E., STADTMAN E. R. STUDY OF THE BIOSYNTHESIS OF PHENAZINE-1-CARBOXYLIC ACID. Arch Biochem Biophys. 1964 Jul 20;106:194–199. doi: 10.1016/0003-9861(64)90175-4. [DOI] [PubMed] [Google Scholar]
- Loper J. E., Haack C., Schroth M. N. Population Dynamics of Soil Pseudomonads in the Rhizosphere of Potato (Solanum tuberosum L.). Appl Environ Microbiol. 1985 Feb;49(2):416–422. doi: 10.1128/aem.49.2.416-422.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola M., Cook R. J. Effects of fungal root pathogens on the population dynamics of biocontrol strains of fluorescent pseudomonads in the wheat rhizosphere. Appl Environ Microbiol. 1991 Aug;57(8):2171–2178. doi: 10.1128/aem.57.8.2171-2178.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M., Bonsall R. F., Pierson L. S. Production of the antibiotic phenazine-1-carboxylic Acid by fluorescent pseudomonas species in the rhizosphere of wheat. Appl Environ Microbiol. 1990 Apr;56(4):908–912. doi: 10.1128/aem.56.4.908-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988 Aug;170(8):3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M., Messenger A. J. Occurrence, biochemistry and physiology of phenazine pigment production. Adv Microb Physiol. 1986;27:211–275. doi: 10.1016/s0065-2911(08)60306-9. [DOI] [PubMed] [Google Scholar]
- Vesper S. J. Production of Pili (Fimbriae) by Pseudomonas fluorescens and Correlation with Attachment to Corn Roots. Appl Environ Microbiol. 1987 Jul;53(7):1397–1405. doi: 10.1128/aem.53.7.1397-1405.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967 Nov;15(6):1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- van Peer Ron, Punte Helma L. M., de Weger Letty A., Schippers Bob. Characterization of Root Surface and Endorhizosphere Pseudomonads in Relation to Their Colonization of Roots. Appl Environ Microbiol. 1990 Aug;56(8):2462–2470. doi: 10.1128/aem.56.8.2462-2470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



