Abstract
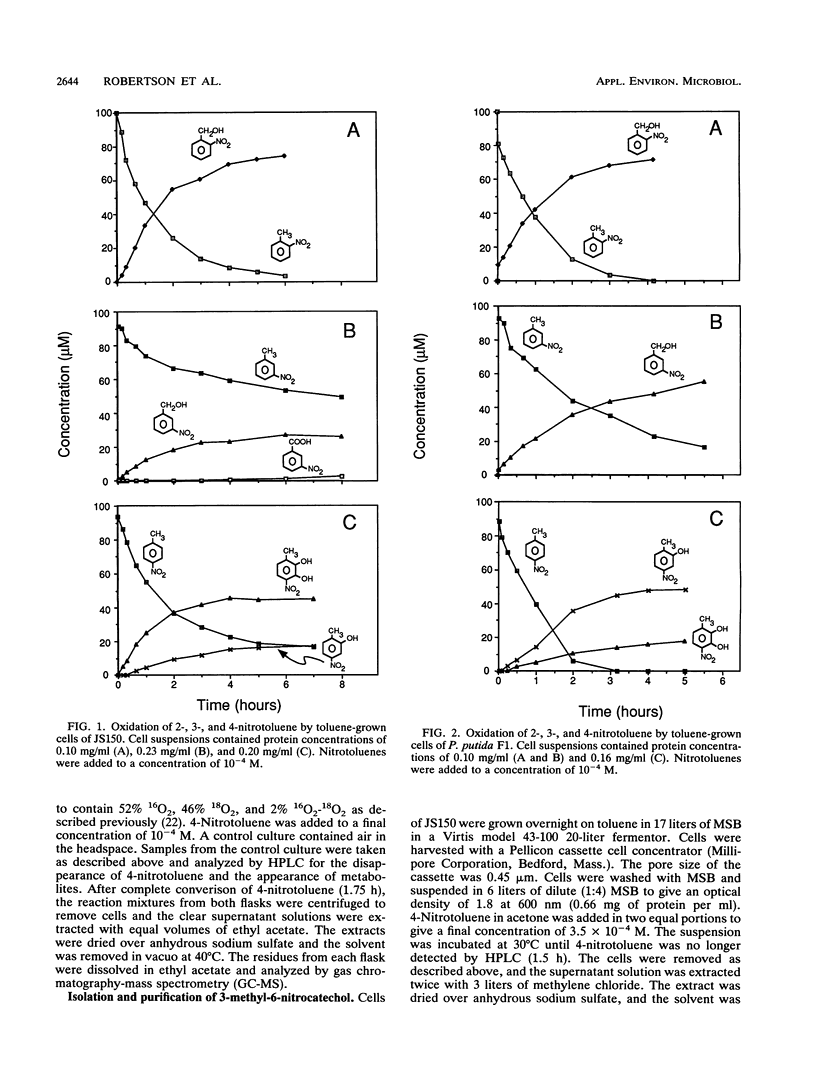
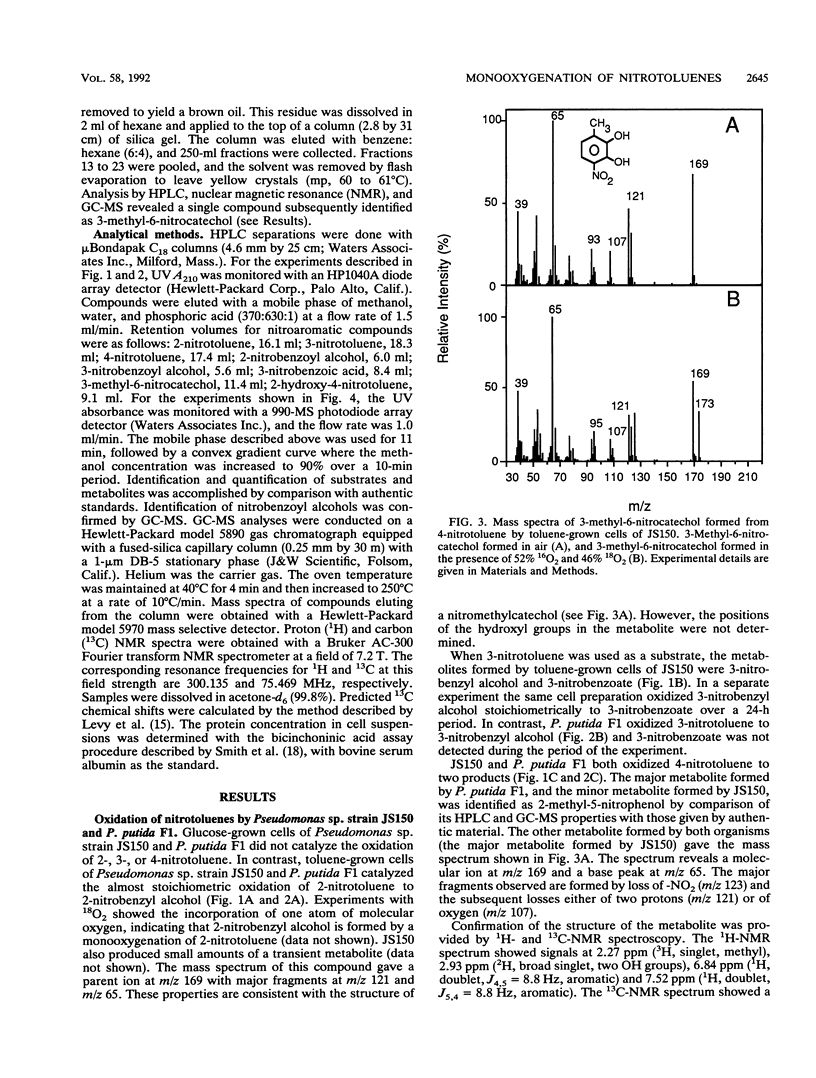
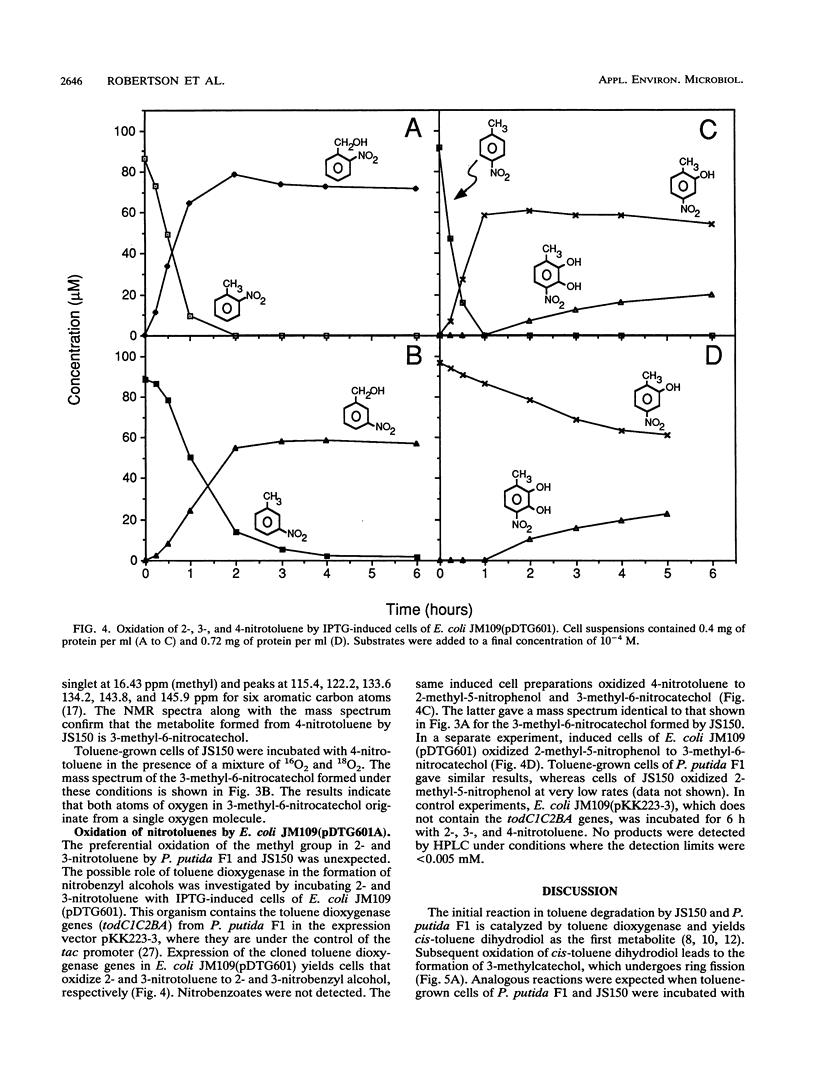
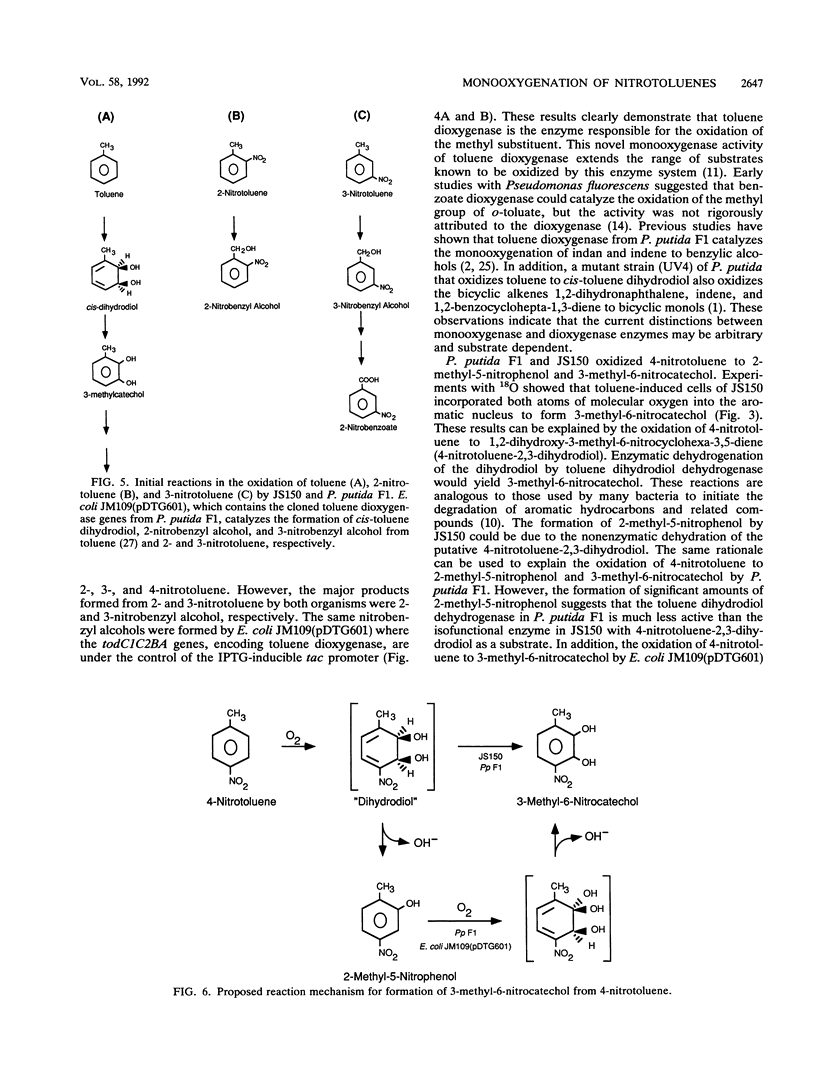
Pseudomonas putida F1 and Pseudomonas sp. strain JS150 initiate toluene degradation by incorporating molecular oxygen into the aromatic nucleus to form cis-1,2-dihydroxy-3-methylcyclohexa-3,5-diene. When toluene-grown cells were incubated with 2- and 3-nitrotoluene, the major products identified were 2- and 3-nitrobenzyl alcohol, respectively. The same cells oxidized 4-nitrotoluene to 2-methyl-5-nitrophenol and 3-methyl-6-nitrocatechol. Escherichia coli JM109(pDTG601), which contains the toluene dioxygenase genes from P. putida F1 under the control of the tac promoter, oxidized the isomeric nitrotoluenes to the same metabolites as those formed by P. putida F1 and Pseudomonas sp. strain JS150. These results extend the range of substrates known to be oxidized by this versatile enzyme and demonstrate for the first time that toluene dioxygenase can oxidize an aromatic methyl substituent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruhn C., Lenke H., Knackmuss H. J. Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl Environ Microbiol. 1987 Jan;53(1):208–210. doi: 10.1128/aem.53.1.208-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIN R. B. The microbial metabolism of nitro-aromatic compounds. J Gen Microbiol. 1958 Aug;19(1):1–14. doi: 10.1099/00221287-19-1-1. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT N. J., CAIN R. B. Bacterial degradation of the nitrobenzoic acids. Biochem J. 1959 Feb;71(2):248–261. doi: 10.1042/bj0710248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado A., Wubbolts M. G., Abril M. A., Ramos J. L. Nitroaromatics Are Substrates for the TOL Plasmid Upper-Pathway Enzymes. Appl Environ Microbiol. 1992 Jan;58(1):415–417. doi: 10.1128/aem.58.1.415-417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968 Jul;7(7):2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Pettigrew C. A., Spain J. C. Biodegradation of mixtures of substituted benzenes by Pseudomonas sp. strain JS150. Appl Environ Microbiol. 1992 Jul;58(7):2237–2244. doi: 10.1128/aem.58.7.2237-2244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallas L. E., Alexander M. Microbial transformation of nitroaromatic compounds in sewage effluent. Appl Environ Microbiol. 1983 Apr;45(4):1234–1241. doi: 10.1128/aem.45.4.1234-1241.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICHIHARA A., ADACHI K., HOSOKAWA K., TAKEDA Y. The enzymatic hydroxylation of aromatic carboxylic acids; substrate specificities of anthranilate and benzoate oxidases. J Biol Chem. 1962 Jul;237:2296–2302. [PubMed] [Google Scholar]
- McCormick N. G., Feeherry F. E., Levinson H. S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976 Jun;31(6):949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Gibson D. T. Oxidation of substituted phenols by Pseudomonas putida F1 and Pseudomonas sp. strain JS6. Appl Environ Microbiol. 1988 Jun;54(6):1399–1404. doi: 10.1128/aem.54.6.1399-1404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Gibson D. T. Pathway for Biodegradation of p-Nitrophenol in a Moraxella sp. Appl Environ Microbiol. 1991 Mar;57(3):812–819. doi: 10.1128/aem.57.3.812-819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Wyss O., Gibson D. T. Enzymatic oxidation of p-nitrophenol. Biochem Biophys Res Commun. 1979 May 28;88(2):634–641. doi: 10.1016/0006-291x(79)92095-3. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Zylstra G. J., Blake C. K., Gibson D. T. Monohydroxylation of phenol and 2,5-dichlorophenol by toluene dioxygenase in Pseudomonas putida F1. Appl Environ Microbiol. 1989 Oct;55(10):2648–2652. doi: 10.1128/aem.55.10.2648-2652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Kwart L. D., Gibson D. T. Benzylic monooxygenation catalyzed by toluene dioxygenase from Pseudomonas putida. Biochemistry. 1988 Feb 23;27(4):1360–1367. doi: 10.1021/bi00404a041. [DOI] [PubMed] [Google Scholar]
- Zylstra G. J., Gibson D. T. Toluene degradation by Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli. J Biol Chem. 1989 Sep 5;264(25):14940–14946. [PubMed] [Google Scholar]


