Abstract
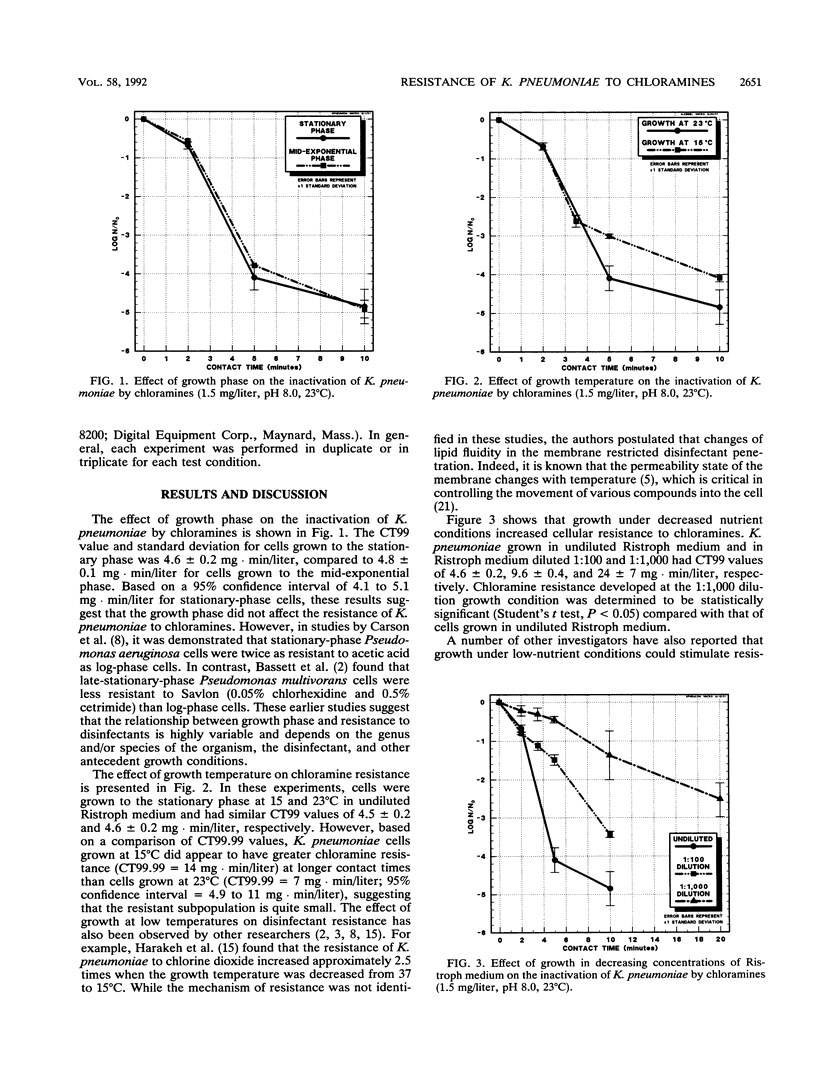
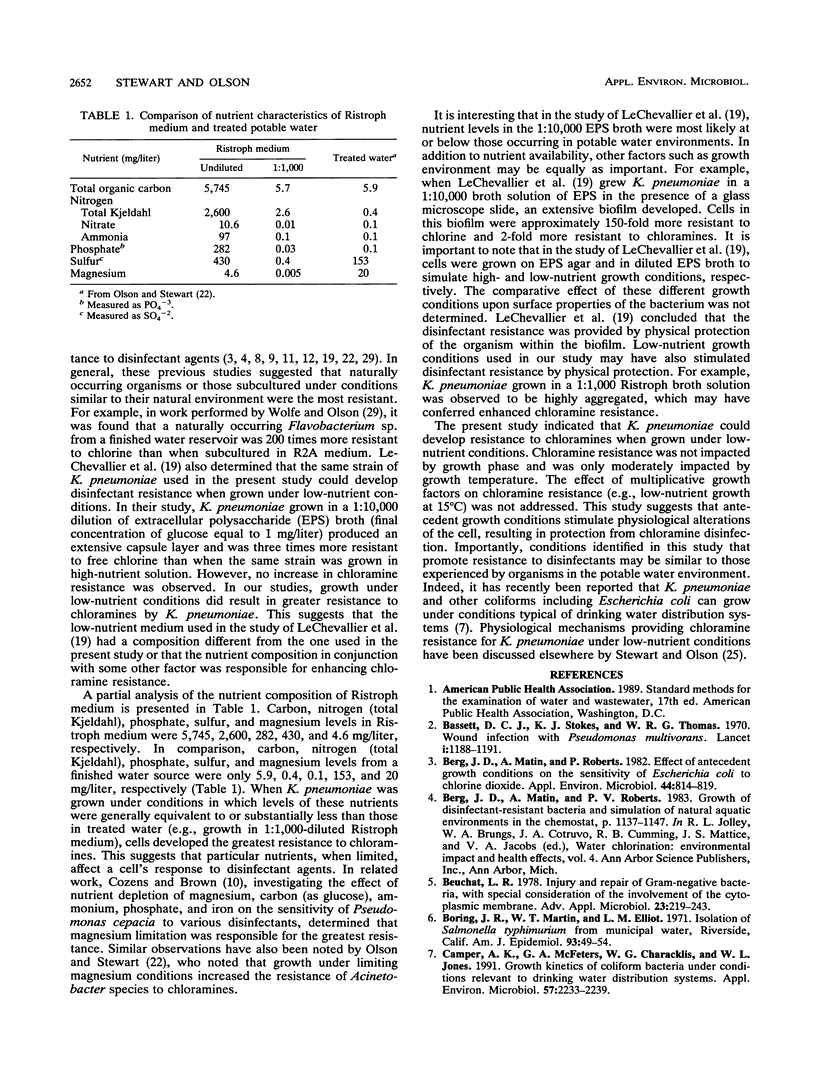
The resistance of Klebsiella pneumoniae to inorganic monochloramine (1.5 mg/liter; 3:1 Cl2:N ratio, pH 8.0) was examined in relation to growth phase, temperature of growth, and growth under decreased nutrient conditions. Growth phase did not impact resistance to chloramines. Mid-exponential and stationary-phase cells, grown in a yeast extract-based medium, had CT99 values and standard deviations of 4.8 +/- 0.1 and 4.6 +/- 0.2 mg.min/liter, respectively. Growth temperature did not alter chloramine resistance at short contact times. CT99 values of cells grown at 15 and 23 degrees C were 4.5 +/- 0.2 and 4.6 +/- 0.2 mg.min/liter, respectively. However, at longer contact times, CT99.99 values of cells grown at 15 and 23 degrees C were 14 and 8 mg.min/liter, respectively, suggesting a small resistant subpopulation for cells grown at the lower temperature. Growth under decreased nutrient conditions resulted in a concomitant increase in resistance to chloramines. When K. pneumoniae was grown in undiluted Ristroph medium and Ristroph medium diluted by 1:100 and 1:1,000, the CT99 values were 4.6 +/- 0.2, 9.6 +/- 0.4, and 24 +/- 7.0 mg.min/liter, respectively. These results indicate that nutrient availability has a greater impact than growth phase or growth temperature in promoting the resistance of K. pneumoniae to inorganic monochloramine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassett D. C., Stokes K. J., Thomas W. R. Wound infection with Pseudomonas multivorans. A water-borne contaminant of disinfectant solutions. Lancet. 1970 Jun 6;1(7658):1188–1191. doi: 10.1016/s0140-6736(70)91783-6. [DOI] [PubMed] [Google Scholar]
- Berg J. D., Matin A., Roberts P. V. Effect of antecedent growth conditions on sensitivity of Escherichia coli to chlorine dioxide. Appl Environ Microbiol. 1982 Oct;44(4):814–819. doi: 10.1128/aem.44.4.814-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuchat L. R. Injury and repair of gram-negative bacteria, with special consideration of the involvement of the cytoplasmic membrane. Adv Appl Microbiol. 1978;23:219–243. doi: 10.1016/s0065-2164(08)70071-6. [DOI] [PubMed] [Google Scholar]
- Boring J. R., 3rd, Martin W. T., Elliott L. M. Isolation of Salmonella typhi-murium from municipal water, Riverside, California, 1965. Am J Epidemiol. 1971 Jan;93(1):49–54. doi: 10.1093/oxfordjournals.aje.a121227. [DOI] [PubMed] [Google Scholar]
- Camper A. K., McFeters G. A., Characklis W. G., Jones W. L. Growth kinetics of coliform bacteria under conditions relevant to drinking water distribution systems. Appl Environ Microbiol. 1991 Aug;57(8):2233–2239. doi: 10.1128/aem.57.8.2233-2239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L. A., Favero M. S., Bond W. W., Petersen N. J. Factors affecting comparative resistance of naturally occurring and subcultured Pseudomonas aeruginosa to disinfectants. Appl Microbiol. 1972 May;23(5):863–869. doi: 10.1128/am.23.5.863-869.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L. A., Petersen N. J., Favero M. S., Aguero S. M. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl Environ Microbiol. 1978 Dec;36(6):839–846. doi: 10.1128/aem.36.6.839-846.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens R. M., Brown M. R. Effect of nutrient depletion on the sensitivity of Pseudomonas cepacia to antimicrobial agents. J Pharm Sci. 1983 Nov;72(11):1363–1365. doi: 10.1002/jps.2600721135. [DOI] [PubMed] [Google Scholar]
- Favero M. S., Carson L. A., Bond W. W., Petersen N. J. Pseudomonas aeruginosa: growth in distilled water from hospitals. Science. 1971 Aug 27;173(3999):836–838. doi: 10.1126/science.173.3999.836. [DOI] [PubMed] [Google Scholar]
- Favero M. S., Drake C. H. Factors influencing the occurrence of high numbers of iodine-resistant bacteria in iodinated swimming pools. Appl Microbiol. 1966 Jul;14(4):627–635. doi: 10.1128/am.14.4.627-635.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh M. S., Berg J. D., Hoff J. C., Matin A. Susceptibility of chemostat-grown Yersinia enterocolitica and Klebsiella pneumoniae to chlorine dioxide. Appl Environ Microbiol. 1985 Jan;49(1):69–72. doi: 10.1128/aem.49.1.69-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff J. C., Akin E. W. Microbial resistance to disinfectants: mechanisms and significance. Environ Health Perspect. 1986 Nov;69:7–13. doi: 10.1289/ehp.86697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamka K. G., LeChevallier M. W., Seidler R. J. Bacterial contamination of drinking water supplies in a modern rural neighborhood. Appl Environ Microbiol. 1980 Apr;39(4):734–738. doi: 10.1128/aem.39.4.734-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Cawthon C. D., Lee R. G. Factors promoting survival of bacteria in chlorinated water supplies. Appl Environ Microbiol. 1988 Mar;54(3):649–654. doi: 10.1128/aem.54.3.649-654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Allen R. G. Liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1980 Jan;11(1):19–21. doi: 10.1128/jcm.11.1.19-21.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuovinen O. H., Hsu J. C. Aerobic and anaerobic microorganisms in tubercles of the Columbus, Ohio, water distribution system. Appl Environ Microbiol. 1982 Sep;44(3):761–764. doi: 10.1128/aem.44.3.761-764.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadowsky R. M., Yee R. B. Satellite growth of Legionella pneumophila with an environmental isolate of Flavobacterium breve. Appl Environ Microbiol. 1983 Dec;46(6):1447–1449. doi: 10.1128/aem.46.6.1447-1449.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


