Abstract
Dynamic assembly and disassembly of microtubules is essential for cell division, cell movements, and intracellular transport. In the developing nervous system, microtubule dynamics play a fundamental role during neurite outgrowth, elongation, and branching, but the molecular mechanisms involved are unknown. SCG10 is a neuron-specific protein that is membrane-associated and highly enriched in growth cones. Here we show that SCG10 binds to microtubules, inhibits their assembly, and can induce microtubule disassembly. We also show that SCG10 overexpression enhances neurite outgrowth in a stably transfected neuronal cell line. These data identify SCG10 as a key regulator of neurite extension through regulation of microtubule instability.
Establishment of a highly specific pattern of connections in the nervous system requires extensive control of nerve fiber growth (1). Neurites must elongate, find the appropriate pathway, branch, and finally establish synapses. Mature connections are also subject to structural rearrangements (2). Growth and remodelling of connections is based on a continuous reorganization of the neuronal cytoskeleton. In axons, one of the main cytoskeletal components is the microtubule (MT), which is oriented with its plus end toward the growth cone. While the minus ends of MTs are relatively stable (3), the plus ends undergo variable phases of assembly and disassembly, also referred to as dynamic instability (4). Drugs that decrease the dynamic behavior of MTs have been found to inhibit neurite extension (5–7). Thus, growth cone advance, and the rate of neurite elongation probably relies on the proper control of assembly and disassembly of MTs. Whereas MT-associated proteins (MAPs) that can stabilize MTs are found in processes and growth cones, factors with the opposite effect have not yet been identified. Recent work (8) has identified the soluble and ubiquitous protein stathmin as a factor that destabilizes MTs by increasing the catastrophe rate (the transition from growing to shrinking) during cell division (9). Interestingly, stathmin is enriched in the developing nervous system (10, 11), but the protein is not detectable in growth cones (unpublished data). SCG10 has sequence homology with stathmin, but the protein is encoded by a different gene (12). SCG10 is neuron-specific, membrane-associated, and concentrated in growth cones (ref. 13 and unpublished data). SCG10 expression is high in the developing nervous system and then dramatically decreases in the adult but persists in regions of synaptic plasticity of the adult brain (11, 14). The levels of SCG10 mRNA are very low in native PC12 cells and in primary chromaffin cells, but they are strongly increased upon nerve growth factor (NGF)-dependent induction of differentiation into sympathetic neurons (15, 16). In PC12 cells, within 12–24 h of NGF-treatment expression of SCG10 mRNA is induced, and by 24–48 h, the amount of SCG10 protein is increased about 6-fold to maximal levels which are maintained in the continuous presence of NGF (16, 17). These correlative data suggest that SCG10 may play a role in neurite outgrowth. However, the specific function of this protein has not yet been elucidated. We analyzed the role of SCG10 in assembly and disassembly of MTs in vitro and determined whether SCG10 overexpression in stably transfected cell lines could affect neurite outgrowth.
MATERIALS AND METHODS
MTs were prepared from porcine cerebrum by three temperature-dependent cycles of cold and warm centrifugations in assembly and disassembly buffer A (0.1 M Mes/1 mM EGTA/0.5 mM MgCl2, pH 6.4). For assembly, 1 mM GTP was added to buffer A (18). This preparation of MTs will be further referred to as “mixed tubulin.” For the isolation of tubulin, MTs were resuspended at a concentration of 20 mg/ml in buffer A, and tubulin was separated from MAPs by an ion exchange chromatography using a 5-ml P11 phosphocellulose column pre-equilibrated with buffer A. MAPs were eluted by a 15-ml gradient of 1 M NaCl in buffer A (19). Protein concentration was determined by Bio-Rad protein assay with bovine serum albumin as standard. The assembly rate of tubulin was measured using a light scattering assay (20, 21). Tubulin or mixed tubulin was used at a concentration of 4 mg/ml. Defined protein amounts and drugs (vinblastine, colcemid, taxol) in 50 μl were mixed with an equal amount of 60% glycerol in buffer A. Absorbance was measured at 350 nm in a Camspec M350 spectrophotometer (Cambridge, U.K.) equipped with seven 50-μl cuvettes and a cooling block for temperature control. In addition, tubulin assembly into MTs was quantified using a sedimentation assay. Samples (80 μl) were taken after 20 min of polymerization at 37°C and overlaid on top of a 150-μl cushion of 60% glycerol in buffer A, and then centrifuged for 30 min at 26,000 × g (30°C). Supernatants were collected, pellets were dissolved in an equal amount of buffer A, and aliquots were prepared for electrophoresis by adding SDS/PAGE sample buffer and boiling (22). Western blot analysis was performed as described (17).
As recombinant full-length SCG10 showed limited solubility and formed aggregates (unpublished data) due to the hydrophobic N-terminal domain of 34 aa, we generated a soluble form of SCG10 that lacks the membrane attachment domain (23). The purified protein was dialyzed against 100 mM Mes/5 mM MgCl2/1 mM EGTA, pH 6.6 at a concentration of 23 μM.
The PC12 cells were grown as described (24). Cells were transfected with a rat cDNA encoding SCG10-8 (ref. 13; generous gift of N. Mori, Research Development Corporation of Japan/PRESTO, Kyoto) cloned in the pcDNA1 Neo Vector (Invitrogen). pcDNA-neo-SCG10 (50 μg) and LipofectAMINE (80 μl) reagent (GIBCO/BRL) were used according to the manufacturer’s protocol. Six days after transfection, cells were subcultured and subjected to selection in growth medium that contained 400 μg/ml G418 (Boehringer Mannheim). After G418 selection for 3–4 weeks, individual colonies were isolated with cloning cylinders, expanded, and frozen in aliquots. To assay SCG10 expression quantitatively, Western blot analysis of PC12 cell extracts with a rabbit antibody to SCG10 was performed in duplicate samples as described (17). As a marker for equal loading, we used the mouse anti-actin monoclonal antibody (C4, Boehringer Mannheim). Cells were also transfected with the vector alone, and G418-resistant clone SCG10-4 was randomly selected. As an additional control, we used nontransfected PC12 cells that were subcloned to select a clone for a vigorous response to NGF. After plating the cells into collagen-coated 96-well multiwell dishes (Biocoat, Collaborative Biomedical Products, Bedford, MA) at a density of 1 cell per well using the Automatic Cell Deposition Unit system of FACStarplus (Becton Dickinson), 16 subclones were scored for neurite outgrowth in response to NGF. Line PC12gg4 was found to respond most robustly in terms of generating neurites of two diameters of the cell after 48 h of NGF exposure. PC12 cells were plated at a density of 10 cells per square centimeter onto poly-d-lysine and laminin-coated coverslips 12 h before the NGF induction. NGF (50 ng/ml) was added, and the cells were fixed after 24 and 48 h and stained with a mouse monoclonal anti-tubulin antibody (Boehringer Mannheim). For quantitative measurement of neurite outgrowth, random fields of cells were analyzed using a semiautomatic computer-assisted image analyzer (Vidas, Kontron, Zurich). After measurement of the surface area of individual neurites and the surface area of the corresponding cell bodies, the ratio of average values was determined (25).
RESULTS AND DISCUSSION
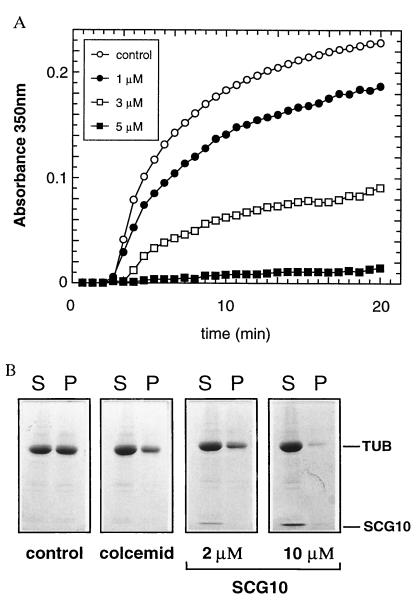
We have found that SCG10 copurified with MTs isolated from juvenile brain and remained associated with the MTs through multiple temperature-dependent polymerization and reassembly cycles (data not shown). To determine whether SCG10 is involved in MT formation, we measured the assembly rate of tubulin using a light scattering assay. Purified tubulin can be induced to undergo cycles of polymerization and depolymerization by switching the temperature of the reaction mixture from 4°C to 37°C and from 37°C to 4°C, respectively. Recombinant soluble SCG10 was able to inhibit MT polymerization in a dose-dependent manner (Fig. 1A). While about 60% inhibition of polymerization was found after addition of 3 μM SCG10, assembly was almost completely abolished by 5 μM SCG10 (molar ratios of SCG10/tubulin dimers were approximately 1:11 and 1:6). After 20 min of polymerization, tubulin assembly into MTs was verified using a sedimentation assay that allows separation of soluble tubulin from assembled tubulin. Fig. 1B shows that SCG10 effectively inhibited tubulin assembly in a dose-dependent manner since an increase in SCG10 concentration resulted in a decrease of polymerized tubulin in the pellet. The inhibition was comparable to that obtained with the potent MT-destabilizing drug colcemid, which inhibits addition of tubulin to MTs similar to colchicine (26, 27). Similar results were obtained with different preparations of tubulin (Figs. 1 and 2). We used either tubulin containing MAPs that copurify with MTs (mixed tubulin) or tubulin separated from MAPs (tubulin). Comparing the effect of SCG10 on MT assembly between the two preparations, we found a small decrease of 20% in the effect of SCG10 when using mixed tubulin. Although this was statistically significant, it indicates that the assembly-promoting effect of MAPs did not efficiently counteract the effect of SCG10.
Figure 1.
Effect of SCG10 on the assembly of MTs. (A) Dose-dependent inhibition of MT assembly by SCG10. Mixed tubulin (tubulin and MAPs, 4 mg/ml) was incubated at 4°C in the absence of SCG10 (control) or in the presence of 1, 3, and 5 μM SCG10. The polymerization was induced by changing the temperature to 37°C at time zero. Determination for each curve was performed in duplicates. The 1, 3, and 5 μM concentrations of SCG10 resulted in inhibitions of about 18%, 60%, and 94%, respectively. (B) In a sedimentation assay, assembled MTs were separated from unpolymerized mixed tubulin by centrifugation through a 60% glycerol cushion. Shown are soluble elements in the supernatant (S) and MTs in pellet (P), separated on an SDS/PAGE gel and stained by Coomassie blue. Equal amounts of mixed tubulin (4 mg/ml) were assembled in buffer only (control) and in the presence of 10 μM colcemid, 2 μM SCG10, and 10 μM SCG10. Only the relevant part of the SDS/PAGE gel is shown. The locations of tubulin and SCG10 are indicated. In the presence of 2 μM SCG10, less tubulin is found in the MT fraction (P) than in the control, and in the presence of 10 μM SCG10, most of the MTs have been depolymerized into soluble tubulin.
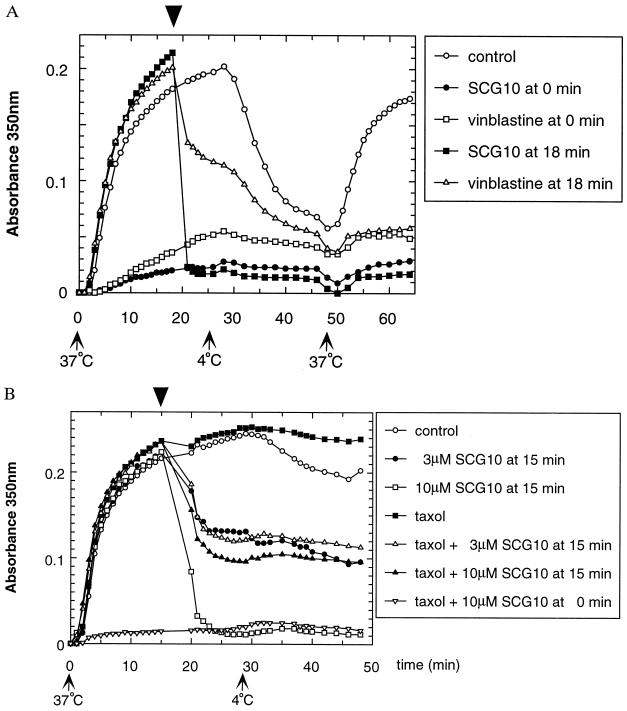
Figure 2.
Effect of SCG10 on MT disassembly. (A) Samples containing 4 mg/ml pure tubulin were allowed to polymerize for 18 min at 37°C. At that time (arrowhead), SCG10 at 8 μM (▪), vinblastine at 4 μM (▵), or buffer as control (○) was added to the samples. SCG10 (•) or vinblastine (□) was also added from the beginning at the same concentrations. Assembly was induced by switching the temperature from 4°C to 37°C (arrow 37°C), and conversely, disassembly was induced by a switch from 37°C to 4°C (arrow 4°C). Experiments were performed at least three times. Here, a representative experiment is shown. SCG10 induced quick and dramatic disassembly of MTs. Absorbance values were immediately decreased to levels obtained when SCG10 was added from the beginning. Control samples showed no important reduction (although in some experiments, a minor decrease was observed). Vinblastine induced some depolymerization but was less efficient than SCG10. Following a switch to 4°C, disassembly occurred as expected in control and vinblastine-treated samples. Finally, following an additional switch to 37°C, tubulin reassembled only in control sample. (B) The effect of SCG10 on taxol-stabilized MTs was studied. Samples containing mixed tubulin at 4 mg/ml were allowed to polymerize for 15 min at 37°C with or without taxol. At that time (arrowhead), SCG10 was added to the samples without taxol at 3 μM (•) or 10 μM (□), and to the samples with taxol (10 μM) at 3 μM (▵) or 10 μM (▴). As a control, buffer was added to MTs alone (○) or to taxol-stabilized MTs (▪). Finally, SCG10 at 10 μM was also added from the beginning in the presence of taxol (▿). Experiments were performed at least three times. Here, a representative experiment is shown. SCG10 was able to inhibit the stabilizing action of taxol partially since about 60% MT disassembly was observed with 10 μM SCG10. A fraction of taxol-stabilized MTs was resistant to SCG10 since 3 μM and 10 μM had a comparable effect on disassembly. When 10 μM SCG10 and taxol were concomitantly added from the beginning, no assembly occurred following the switch to 37°C, suggesting that taxol was unable to compensate for the inhibitory effect of SCG10. Note that in the absence of taxol, 50% disassembly was induced by 3 μM SCG10 and almost complete depolymerization was observed with 10 μM SCG10.
To investigate whether SCG10 is able to induce depolymerization of assembled MTs, the protein was added to the reaction mixture after 18 minutes of polymerization at 37°C (Fig. 2A). SCG10 induced a rapid and dramatic disassembly of MTs at a concentration of 8 μM, and the protein had a faster and more potent action than the depolymerizing drug vinblastine (Fig. 2A). This drug acts on assembled MTs in the micromolar concentration range (28–30). Following a temperature switch to 4°C to depolymerize MTs in all samples, another cycle of assembly was induced by raising the temperature to 37°C. Tubulin reassembled in the control sample but not in the SCG10- or vinblastine-treated samples, consistent with an inhibitory effect on polymerization (Fig. 2A). Similar experiments were performed in the presence of taxol to assess whether SCG10 could antagonize the MT-stabilizing effect of the drug at a concentration of 10 μM (31, 32). Fig. 2B shows that SCG10 was able to induce disassembly of taxol-stabilized MTs. However, the disassembly was only partial, and no major difference was found for both concentrations of SCG10 (3 and 10 μM; a maximum of 40% of MTs remained assembled). This suggests that a fraction of taxol-stabilized MTs were resistant to SCG10-induced depolymerization. In summary, these data indicate that SCG10 has potent activity in preventing tubulin assembly and inducing MT disassembly. Its preferential localization in neuronal growth cones (ref. 13 and unpublished data) strongly suggests that the relative levels of SCG10 regulate MT dynamics in this highly motile cellular compartment.
If this hypothesis is correct, SCG10 should regulate neurite outgrowth by its action on tubulin assembly in the growth cones of differentiating neurons. Consistent with this, high levels of SCG10 expression are correlated with periods of neurite elongation (13, 14). To test the role of SCG10 in neurite outgrowth, we generated stable PC12 cell lines constitutively expressing SCG10. Two cell lines that expressed the highest amount of SCG10 (SCG10-A and SCG10-B) were further analyzed. In addition, we established control lines (SCG10-4 and PC12gg4). Both SCG10-overexpressing lines expressed similar levels of SCG10, as determined by Western blotting. The amount of SCG10 protein was determined before each experiment and was consistently ca. 10-fold higher (data not shown) in the SCG10-overexpressing lines than that in cells transfected with the vector alone or in the nontransfected PC12 subclone gg4. The overexpressing cells showed the expected perinuclear localization of SCG10 in the area of the Golgi complex (ref. 13 and unpublished data) and, after induction of neuronal differentiation, in the growth cones (Fig. 3). No neurite outgrowth was observed in SCG10-overexpressing lines in the absence of NGF, indicating that constitutive expression of SCG10 at high levels is not sufficient to induce neurite outgrowth. Upon NGF induction, the SCG10-transfected lines showed a dramatic increase in the tendency to elongate neurites relative to the control cells (Figs. 3 and 4). In two independent experiments, 38 ± 2% of the cells overexpressing SCG10 extended processes after 2 days of NGF exposure, whereas only 10 ± 2% of control cells had neurites (Fig. 4A). In addition to the increase in the number of cells with neurites, we observed a remarkable increase in the lengths of the processes in cells overexpressing SCG10 as compared with the control cells. To measure neurite outgrowth, the four cell lines were exposed to NGF for 1 and 2 days. SCG10-overexpressing cells had on average neurites 2.6 times longer than those of the controls (Fig. 4B), indicating that SCG10 overexpression enhances neurite elongation. We had previously showed that the related protein stathmin is an effector molecule in the NGF signaling pathway (17). It is, however, unlikely that the effect of SCG10 overexpression on neurite outgrowth is due to a similar signaling function in the differentiation pathway. Stathmin-depleted PC12 cells induce SCG10 protein normally upon NGF treatment but do not differentiate, suggesting that SCG10 cannot substitute stathmin function. The preferential localization of SCG10 in the growth cone and its action to disassemble MTs in vitro rather suggests that SCG10 overexpression enhances neurite outgrowth by a direct effect on MTs in the growth cones. A highly dynamic state of MT polymer in the growth cone, with transitions between MT growth and shrinkage, appears to be essential for growth cone advance; drugs that either stabilize (e.g., taxol) or destabilize (e.g., vinblastine) MTs inhibit neurite extension (5–7). The neurite shaft of differentiated PC12 cells contains mainly stabilized MTs, whereas MTs in the growth cone are particularly labile (33, 34), as has been reported for axons of neurons (35, 36). The highly dynamic behavior of MT polymers in the growth cone cannot be explained solely by the activities of the characterized MAPs, which stabilize and promote MT assembly (36), but this behavior implies that there are additional factors that promote the disassembly of MTs. Our data suggest that SCG10 is such a cellular factor that promotes neurite outgrowth by increasing the dynamic instability of MTs in the growth cone.
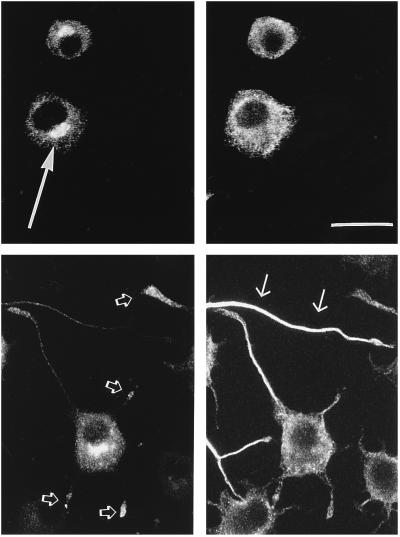
Figure 3.
Double-immunofluorescence of control (Upper) and SCG10-transfected (Lower) PC12 cells after 24 h of NGF treatment. Cells were double-stained for SCG10 (Left) and tubulin (Right). Control cells had generally no neurites after 24 h of NGF induction and showed mainly Golgi localization of SCG10 (large arrow). Cells transfected with SCG10 had tubulin-immunopositive neurites (small arrows), and in addition to the Golgi localization, SCG10 was observed in the growth cones of outgrowing neurites (open arrows). Representative cells are shown. Cells were plated on poly-d-lysine- and laminine-coated coverslips and fixed in 4% formaldehyde after 24 h incubation in NGF-containing medium. Coverslips were incubated with a rabbit anti-SCG10 antiserum (17) and a mouse anti-tubulin antibody (Boehringer Mannheim) in buffer containing 10% rat serum, 0.3% Triton X-100, and 2% bovine serum albumin overnight at 4°C. Appropriate secondary antibodies labeled with either fluorescein or Texas Red (Vector Laboratories) were used to detect immune complexes. (Bar = 25 μM.)
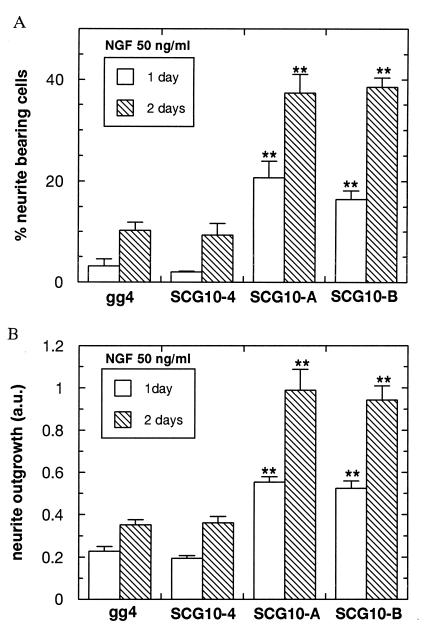
Figure 4.
(A) Overexpression of SCG10 in PC12 cells causes an increase in the number of neurite-bearing cells in response to NGF. The percentage of cells with neurites in SCG10-transfected (SCG10-A and SCG10-B) and control lines (gg4 and SCG10-4) was assayed after 24 h (open bars) and after 48 h (hatched bars) of NGF induction. Neurites were scored as processes greater than two cell diameters in length. The percentage of neurite-bearing cells was determined as the average of three counts on at least 100 cells. The proportion of the cells with neurites in the SCG10-overexpressing cell lines was significantly greater than in controls (∗∗, P < 0.001 for SCG10-A and P < 0.01 for SCG10-B). (B) Effect of SCG10 overexpression on neurite outgrowth. PC12 cells of the control (gg4 and SCG10-4) and SCG10-overexpressing lines (SCG10-A and SCG10-B) were induced with NGF for 24 h (open bars) and 48 h (hatched bars). Neurite outgrowth was quantitatively measured using an semiautomatic computer-assisted image analyzer (25). Values are the mean ± SEM of three independent experiments and represent neurite outgrowth defined as the ratio between the surface area of neurites and the surface area of cell bodies of at least 100 cells each. Neurites of the SCG10 lines extend significantly longer than those of the control lines (∗∗, P < 0.001 for SCG10-A and SCG10-B).
Acknowledgments
We gratefully acknowledge the assistance of V. Sommaro for MT preparation and S. Montessuit for purification of SCG10 protein. We thank J.-P. Aubry, H. Blasey, and A. Osen-Sand for their help at various stages of this work. We also thank H. Betz, G. Innocenti, J. Kirsch, and J. Staple for critical comments on the manuscript. V.P. was supported by a postdoctoral fellowship from the Conseil Régional Rhône-Alpes, and B.M.R. was supported by Grant 31-43137.95 of the National Science Foundation of Switzerland.
Footnotes
Abbreviations: MT, microtubule; MAP, MT-associated protein; NGF, nerve growth factor.
References
- 1.Goodman, C. S. & Shatz, C. J. (1993) Cell 72, Suppl., 77–98. [DOI] [PubMed]
- 2.Catsicas S, Grenningloh G, Merlo Pich E. Trends Neurosci. 1994;17:368–373. doi: 10.1016/0166-2236(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 3.Baas P W, Black M M. J Cell Biol. 1990;111:495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchison T, Kirschner M. Nature (London) 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 5.Letourneau P C, Ressler A H. J Cell Biol. 1984;98:1355–1362. doi: 10.1083/jcb.98.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka E, Ho T, Kirschner M W. J Cell Biol. 1995;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochlin M W, Wickline K M, Bridgman P C. J Neurosci. 1996;16:3236–3246. doi: 10.1523/JNEUROSCI.16-10-03236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmont L D, Mitchison T J. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 9.Belmont L D, Hyman A A, Sawin K E, Mitchison T J. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- 10.Amat J A, Fields K L, Schubart U K. Dev Brain Res. 1991;60:205–218. doi: 10.1016/0165-3806(91)90049-o. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura Y, Mori N. Dev Brain Res. 1996;90:73–91. doi: 10.1016/0165-3806(96)83488-2. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Yoshida B N, Avraham K B, Wang H, Wuenschell C W, Jenkins N A, Copeland N G, Anderson D J, Mori N. Genomics. 1993;18:360–373. doi: 10.1006/geno.1993.1477. [DOI] [PubMed] [Google Scholar]
- 13.Stein R, Mori N, Matthews K, Lo L C, Anderson D J. Neuron. 1988;1:463–476. doi: 10.1016/0896-6273(88)90177-8. [DOI] [PubMed] [Google Scholar]
- 14.Himi T, Okazaki T, Wang H, McNeill T H, Mori N. Neuroscience. 1994;60:907–926. doi: 10.1016/0306-4522(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 15.Anderson D J, Axel R. Cell. 1985;42:649–662. doi: 10.1016/0092-8674(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 16.Stein R, Orit S, Anderson D J. Dev Biol. 1988;127:316–325. doi: 10.1016/0012-1606(88)90318-1. [DOI] [PubMed] [Google Scholar]
- 17.Di Paolo G, Pellier V, Catsicas M, Antonsson M, Catsicas S, Grenningloh G. J Cell Biol. 1996;133:1383–1390. doi: 10.1083/jcb.133.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karr L, White H D, Purich D L. J Biol Chem. 1979;254:6107–6111. [PubMed] [Google Scholar]
- 19.Weingarten M D, Lockwood A H, Hwo S-Y, Kirschner M W. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riederer B, Cohen R, Matus A. J Neurocytol. 1986;15:763–775. doi: 10.1007/BF01625193. [DOI] [PubMed] [Google Scholar]
- 21.Herzog W, Weber K. Eur J Biochem. 1978;92:1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- 22.Riederer B M, Goodman S R. FEBS Lett. 1990;277:49–52. doi: 10.1016/0014-5793(90)80807-u. [DOI] [PubMed] [Google Scholar]
- 23.Antonsson, B., Montesuit, S., Di Paolo, G., Lütjens, R. & Grenningloh, G. (1997) Protein Expression Purif., in press. [DOI] [PubMed]
- 24.Greene L A, Tischler A S. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osen-Sand A, Catsicas M, Staple J, Jones K A, Ayala G, Knowles J, Grenningloh G, Catsicas S. Nature (London) 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- 26.Hastie S B. Pharmacol Ther. 1991;51:377–401. doi: 10.1016/0163-7258(91)90067-v. [DOI] [PubMed] [Google Scholar]
- 27.Bershadsky A D, Vasiliev J M. In: Cytoskeleton. Siekevitz P, editor. New York: Plenum; 1988. pp. 1–298. [Google Scholar]
- 28.Prasad V, Jordan M A, Luduena R F J. Protein Chem. 1992;11:509–515. doi: 10.1007/BF01025028. [DOI] [PubMed] [Google Scholar]
- 29.Jordan M A, Thrower D, Wilson L. J Cell Sci. 1992;102:401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- 30.Vallee R B. J Cell Biol. 1982;92:435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnal I, Wade R H. Curr Biol. 1995;5:900–908. doi: 10.1016/s0960-9822(95)00180-1. [DOI] [PubMed] [Google Scholar]
- 32.Okabe S, Hirokawa N. J Cell Biol. 1988;107:651–664. doi: 10.1083/jcb.107.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim S S, Sammak P J, Borisy G. J Cell Biol. 1989;109:253–263. doi: 10.1083/jcb.109.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamburg J R, Bray D, Chapman K. Nature (London) 1986;321:788–790. doi: 10.1038/321788a0. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka E, Kirschner K. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirokawa N. Curr Opin Cell Biol. 1994;6:74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]