Abstract
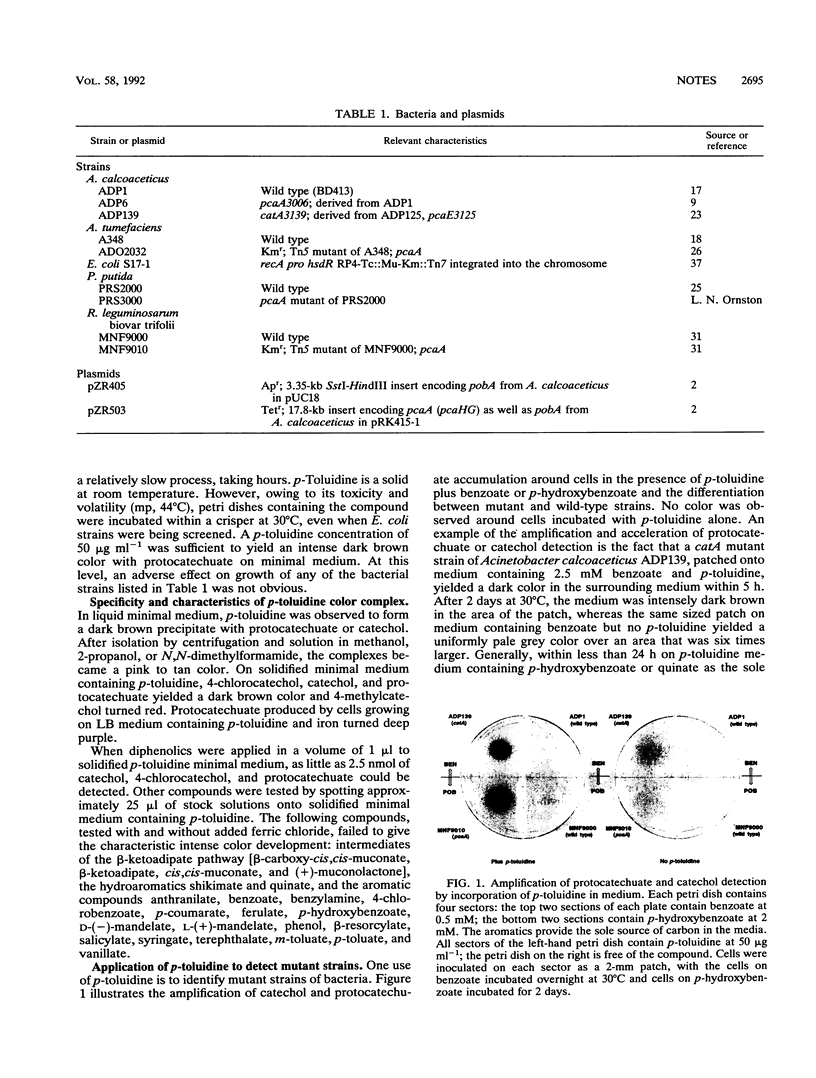
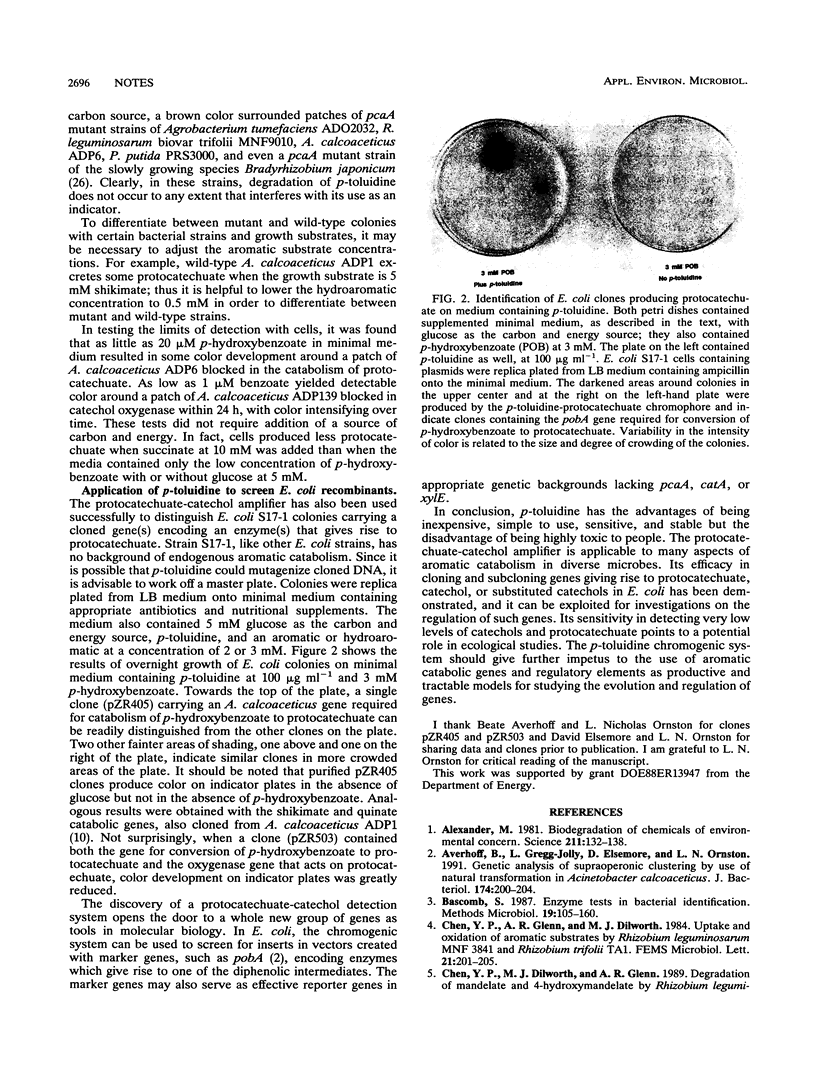
In the presence of p-toluidine and iron, protocatechuate and catechols yield color. Inclusion of p-toluidine in media facilitates the screening of microbial strains for alterations affecting aromatic catabolism. Such strains include mutants affected in the expression of oxygenases and Escherichia coli colonies carrying cloned or subcloned aromatic catabolic genes which encode enzymes giving rise to protocatechuate or catechol. The diphenolic detection system can also be applied to the creation of vectors relying on insertion of cloned DNA into one of the latter marker genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981 Jan 9;211(4478):132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- Averhoff B., Gregg-Jolly L., Elsemore D., Ornston L. N. Genetic analysis of supraoperonic clustering by use of natural transformation in Acinetobacter calcoaceticus. J Bacteriol. 1992 Jan;174(1):200–204. doi: 10.1128/jb.174.1.200-204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A. L. Industrial microbiology. Science. 1981 Nov 27;214(4524):987–995. doi: 10.1126/science.6946560. [DOI] [PubMed] [Google Scholar]
- Doten R. C., Ngai K. L., Mitchell D. J., Ornston L. N. Cloning and genetic organization of the pca gene cluster from Acinetobacter calcoaceticus. J Bacteriol. 1987 Jul;169(7):3168–3174. doi: 10.1128/jb.169.7.3168-3174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Microbial degradation of halogenated compounds. Science. 1985 Apr 12;228(4696):135–142. doi: 10.1126/science.228.4696.135. [DOI] [PubMed] [Google Scholar]
- Gibson D. T. Microbial degradation of aromatic compounds. Science. 1967 Sep 13;161(3846):1093–1097. [PubMed] [Google Scholar]
- Grant S., Roberts C. F., Lamb H., Stout M., Hawkins A. R. Genetic regulation of the quinic acid utilization (QUT) gene cluster in Aspergillus nidulans. J Gen Microbiol. 1988 Feb;134(2):347–358. doi: 10.1099/00221287-134-2-347. [DOI] [PubMed] [Google Scholar]
- Hungria M., Joseph C. M., Phillips D. A. Rhizobium nod Gene Inducers Exuded Naturally from Roots of Common Bean (Phaseolus vulgaris L.). Plant Physiol. 1991 Oct;97(2):759–764. doi: 10.1104/pp.97.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram C., Brawner M., Youngman P., Westpheling J. xylE functions as an efficient reporter gene in Streptomyces spp.: use for the study of galP1, a catabolite-controlled promoter. J Bacteriol. 1989 Dec;171(12):6617–6624. doi: 10.1128/jb.171.12.6617-6624.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Lehrbach P. R., Timmis K. N. Genetic analysis and manipulation of catabolic pathways in Pseudomonas. Biochem Soc Symp. 1983;48:191–219. [PubMed] [Google Scholar]
- Manafi M., Kneifel W., Bascomb S. Fluorogenic and chromogenic substrates used in bacterial diagnostics. Microbiol Rev. 1991 Sep;55(3):335–348. doi: 10.1128/mr.55.3.335-348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C. A., Hartwig U. A., Joseph C. M., Phillips D. A. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce nod Genes of Rhizobium meliloti. Plant Physiol. 1989 Nov;91(3):842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. Properties of an inducible uptake system for beta-ketoadipate in Pseudomonas putida. J Bacteriol. 1976 Feb;125(2):475–488. doi: 10.1128/jb.125.2.475-488.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D., Ornston L. N. Enzymes of the beta-ketoadipate pathway are inducible in Rhizobium and Agrobacterium spp. and constitutive in Bradyrhizobium spp. J Bacteriol. 1986 Jan;165(1):288–292. doi: 10.1128/jb.165.1.288-292.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D., Ornston L. N., Nester E. W. Chemotaxis to plant phenolic inducers of virulence genes is constitutively expressed in the absence of the Ti plasmid in Agrobacterium tumefaciens. J Bacteriol. 1987 Nov;169(11):5336–5338. doi: 10.1128/jb.169.11.5336-5338.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D., Rivelli M., Ornston L. N. Chemotaxis to aromatic and hydroaromatic acids: comparison of Bradyrhizobium japonicum and Rhizobium trifolii. J Bacteriol. 1985 Aug;163(2):417–422. doi: 10.1128/jb.163.2.417-422.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D., Rynne F., Glenn A. Regulation of phenolic catabolism in Rhizobium leguminosarum biovar trifolii. J Bacteriol. 1991 Sep;173(17):5546–5550. doi: 10.1128/jb.173.17.5546-5550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Verma D. P. Phenolic compounds as regulators of gene expression in plant-microbe relations. Mol Plant Microbe Interact. 1990 Jan-Feb;3(1):4–8. doi: 10.1094/mpmi-3-004. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukowski M. M., Gaffney D. F., Speck D., Kauffmann M., Findeli A., Wisecup A., Lecocq J. P. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1101–1105. doi: 10.1073/pnas.80.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]




