Abstract
Prostaglandins (PGs) function as intracellular signal mediators in the regulation of a variety of physiological and pathological processes, including inflammation and immune responses. Cyclopentenone PGs are characterized by antiviral activity against several viruses, including human immunodeficiency virus type 1 (HIV-1), and by the ability to induce heat shock protein expression through activation of the heat shock transcription factor. Here we report that PGA1 is a potent inhibitor of nuclear factor-κB (NF-κB) activation in human cells and of NF-κB-dependent HIV-1 transcription in long terminal repeat-chloramphenicol acetyltransferase transient transfection experiments. PGA1 acts by inhibiting phosphorylation and preventing degradation of the NF-κB inhibitor IκB-α. Inhibition does not require protein synthesis, is dependent on the presence of a reactive cyclopentenonic moiety, and is associated with heat shock transcription factor activation. Because NF-κB is critically involved in the activation of immunoregulatory and viral genes, inhibition of its activity could be a major component of the immunosuppressive and antiviral activity of PGs.
Keywords: antiviral, HIV, IκB-α, tumor necrosis factor α
Nuclear factor-κB (NF-κB), an inducible eukaryotic transcription factor of the rel family, normally exists in an inactive cytoplasmic complex, whose predominant form is a heterodimer composed of p50 and p65 (Rel A) subunits, bound to inhibitory proteins of the IκB family, and is activated in response to primary (viruses, bacteria, UV) or secondary (inflammatory cytokines) pathogenic stimuli (1, 2). Stimulation triggers the release of NF-κB from IκB, resulting in NF-κB translocation to the nucleus, where it binds to DNA at specific κB sites, rapidly inducing a variety of genes encoding signaling proteins (2). NF-κB is considered an immediate early mediator of immune and inflammatory responses and is involved in many pathological events, including progression of AIDS, by enhancing the transcription of human immunodeficiency virus type 1 (HIV-1) (3, 4).
Prostaglandins (PGs) are a class of naturally occurring cyclic 20-carbon fatty acids with potent biological properties. In eukaryotic cells, they are synthesized from arachidonic acid (AA) and other polyunsaturated fatty acid precursors derived from the phospholipid pool of the cell membrane in response to external stimuli (5), and function as intracellular signal mediators in the regulation of physiological and pathological processes, including inflammation (6), immune responses (7), febrile responses (8), cytoprotection (9), and cell proliferation and differentiation (10). PGs are also involved in the control of virus replication (11). Starting from the early observation that PGs of the A type (PGAs) inhibit paramyxovirus replication (12), it is now well established that PGs containing an α,β-unsaturated carbonyl group in the cyclopentane ring structure (cyclopentenone PGs; i.e., PGAs and PGJs) inhibit the replication of a wide variety of DNA and RNA viruses, including HIV-1 (reviewed in ref. 11). In HIV-1-infected cells, cyclopentenone PGs were recently shown to act by selectively blocking HIV-1 mRNA transcription (13). In negative strand RNA viruses, the antiviral activity is mediated by selective inhibition of viral protein synthesis and maturation, which has been associated with the turning on of an intracellular defense response and induction of heat shock protein synthesis (14, 15). Cyclopentenone PGs are in fact characterized by the ability to selectively induce the expression of specific heat shock genes in a nonstressful situation in mammalian cells (16). Induction is mediated by the activation and translocation to the nucleus of a transregulatory protein, the heat shock transcription factor (HSF), which binds to multiple arrays of heat shock elements (HSEs) located in the promoter of heat shock genes (17). Several HSFs (HSF1–HSF4) have been identified in vertebrate cells (18). In mammalian cells, HSFs exist as an inactive non-DNA-binding form, which is rapidly converted to the DNA-binding form upon exposure to heat shock or other environmental stimuli (18). As described for NF-κB, HSF activation is also a multistep process. In the case of HSF, activation requires oligomerization, acquisition of DNA-binding activity, localization to the nucleus, and phosphorylation (18).
We now report that PGA1 and other cyclopentenone PGs inhibit the activation of NF-κB in human cells. Inhibition of NF-κB activation is associated with induction of HSF, and it results in selective inhibition of κB-controlled transcriptional activation of HIV-1 long terminal repeat (LTR) reporter genes.
METHODS
Cell Culture.
Jurkat T cells, CEM-SS T lymphoid cells, and HeLa cells were grown in RPMI 1640 medium (Jurkat and CEM-SS cells) or DMEM (HeLa cells), supplemented with 10% fetal calf serum (FCS) and antibiotics at 37°C in a 5% CO2 atmosphere. Cells (106 per ml) were stimulated with 25 ng/ml 12-O-tetradecanoylphorbol 13-acetate (TPA) (Sigma) or 10 ng/ml recombinant human tumor necrosis factor α (TNF-α) (Sigma), in the presence of PGA1 (Cayman Chemicals, Ann Arbor, MI) or ethanol diluent. Unless specified differently, cells were treated with PGA1 (24 μM) in RPMI 1640 medium containing 10% FCS starting 2 h before the addition of TPA. TPA was kept in the culture medium for the duration of the experiment. Stimulation was stopped by cooling on ice and immediate centrifugation of cells in an Eppendorf microfuge.
Electrophoretic Mobility-Shift Assay (EMSA).
Whole-cell extracts were prepared after lysis in a high-salt extraction buffer containing 20 mM Hepes (pH 7.9), 0.35 M NaCl, 20% glycerol, 1% Nonidet P-40, 1 mM MgCl2, 1 mM DTT, 0.5 mM EDTA, 0.1 mM EGTA, 0.2% aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.5 μg/ml leupeptin, and 0.7 μg/ml pepstatin (19). Nuclear and cytoplasmic extracts were prepared as described (20). Aliquots of total, nuclear, or cytoplasmic extracts (15, 5, or 15 μg of protein, respectively) were incubated with a 32P-labeled κB DNA probe (21) or a HSE probe (22), followed by analysis of DNA-binding activities by EMSA. Binding reactions were performed as described (22). Complexes were analyzed by nondenaturing 4% PAGE. Specificity of protein–DNA complexes was verified by immunoreactivity with polyclonal antibodies specific for p65 (Rel A) or HSF-1, for NF-κB and HSF, respectively. Quantitative evaluation of NF-κB–κB and HSF–HSE complex formation was determined by Molecular Dynamics PhosphorImager analysis.
Western Blot Analysis.
Equal amounts of protein (50 μg per sample) in whole-cell extracts were separated by SDS/PAGE, blotted to nitrocellulose, and filters were incubated with polyclonal anti-IκB-α/MAD3 antibodies (Santa Cruz Biotechnology) or rabbit polyclonal anti-HSF1 (kindly provided by R. I. Morimoto, Northwestern University, Evanston, IL) followed by decoration with peroxidase-labeled anti-rabbit IgG (ECL, Amersham), as described (16). For phosphatase treatment, whole-cell extracts (50 μg per sample) were resuspended in 25 μl reaction mixture containing 2.5 μl of 10× phosphatase buffer (500 mM Tris·Cl, pH 9.0/10 mM MgCl2) and 12 units of calf intestinal phosphatase in the presence or absence of the phosphatase inhibitor NaH2PO4 (10 mM, pH 9.0) and incubated for 1 h at 37°C before processing for Western blot analysis, as described (23).
Transfection and Chloramphenicol Acetyltransferase (CAT) Assay.
The HIV-LTR wt and HIV-LTR κB mu constructs (4), which contain, respectively, the wild type (wt) or the mutated (mu) HIV enhancer and promoter regions upstream of the bacterial CAT gene, were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. The human HSP70-CAT construct LSN wt (24) was a kind gift of R. I. Morimoto. Jurkat cells (107) were transfected with 10 μg of HIV-LTR wt or HIV-LTR κB mu, and 12 μg of LSN wt constructs using a DEAE-dextran method, as described (25). Cells were maintained in RPMI 1640 medium containing 10% FCS for 24 h before TPA stimulation. Cell extracts were prepared for CAT assay, and CAT activity was measured by determining the amount of acetylated chloramphenicol produced from 1-deoxy-[dichloroacetyl-1-14C]-chloramphenicol. CAT activity was quantified by standard methods, as described (26).
RESULTS AND DISCUSSION
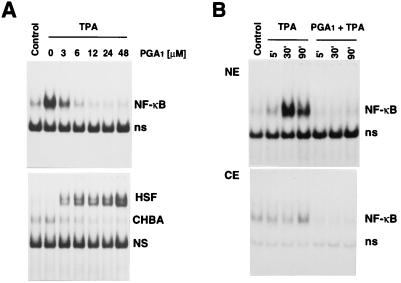
Human Jurkat T cells were treated with the NF-κB inducer TPA alone or in the presence of PGA1. Whole-cell, cytoplasmic, or nuclear extracts were analyzed by EMSA to determine NF-κB and HSF activation. TPA stimulated NF-κB activation, whereas it did not induce HSF. PGA1 was found to inhibit TPA-induced NF-κB activation, while it activated HSF in a dose-dependent manner (Fig. 1). Under the conditions described, NF-κB was activated for at least 24 h in the presence of TPA; inhibition of NF-κB activation by PGA1 was maximal up to 9 h and decreased 24 h after TPA treatment concomitantly with the decrease in HSF activation (data not shown). In unstimulated cells PGA1 had no effect on NF-κB.
Figure 1.
Dose-dependent effect of PGA1 on DNA-binding activity of NF-κB and HSF transcription factors. (A) Jurkat cells exposed to PGA1 (3–48 μM) or ethanol diluent in FCS-free RPMI 1640 medium for 2 h were treated with TPA (25 ng/ml). Control cells were neither stimulated nor treated with PGA1. After 3 h whole-cell extracts from control or TPA-treated cells were incubated with 32P-labeled κB DNA (Upper) or HSE (Lower) probes, followed by analysis of DNA-binding activities by EMSA. Sections of fluorograms from native gels are shown. Position of NF-κB–DNA complex (NF-κB) and nonspecific binding (ns) are indicated (Upper). Position of HSF–DNA binding complex (HSF), constitutive HSE-binding activity (CHBA) (22), and nonspecific protein–DNA interactions (NS) are shown (Lower). (B) Nuclear (NE) (Upper) and cytoplasmic (CE) (Lower) extracts of Jurkat cells treated with TPA in the presence or the absence of PGA1 (24 μM) were prepared at different times after TPA addition and subjected to EMSA, as described above.
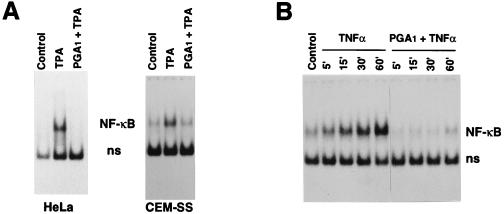
Inhibition of NF-κB activation by PGA1 is not specific for Jurkat cells or for TPA induction. PGA1 was equally effective in inhibiting NF-κB activation in HeLa cells and CEM-SS lymphoid cells (Fig. 2A), as well as human monocytes from healthy donors (G.E. and M.G.S., unpublished data). NF-κB activation by TNF-α was also inhibited by PGA1 (Fig. 2B). In all types of cells examined, NF-κB inhibition could be obtained at concentrations that did not inhibit nucleic acid or protein synthesis, and was associated with HSF activation (data not shown).
Figure 2.
PGA1 inhibits NF-κB activation by TPA or TNF-α in different types of human cells. (A) Inhibition of TPA-induced NF-κB activation by PGA1 in HeLa or in CEM-SS cells. Nuclear extracts from HeLa or CEM-SS cells unstimulated (Control) or stimulated with TPA (25 ng/ml) for 2 h in the presence or absence of PGA1 (24 μM for HeLa cells, 12 μM for CEM-SS cells), were subjected to EMSA for determination of NF-κB activation. (B) Inhibition of TNF-α-induced activation of NF-κB in Jurkat cells. TNF-α (10 ng/ml) was added to cells treated with PGA1 (24 μM) for 2 h. Whole-cell extracts were prepared at different times after addition of TNF-α and subjected to EMSA for determination of NF-κB activation, as described above. Sections of fluorograms from native gels are shown. Position of NF-κB–DNA complex (NF-κB) and nonspecific binding (ns) are indicated.
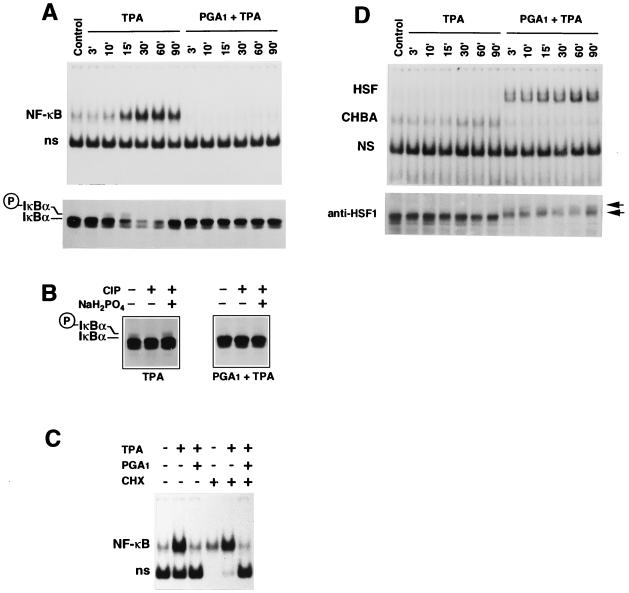
NF-κB activation is dependent on the phosphorylation and degradation of IκB-α (27); however, the mechanism of NF-κB activation via IκB phosphorylation has not been completely elucidated. In Jurkat cells, TPA induced NF-κB activation by ≈15 min after treatment (Fig. 3A Upper). At this time, the amount of IκB-α protein was notably reduced in TPA-stimulated cells (Fig. 3A Lower). As described, ≈60-90 min after TPA stimulation the level of IκB-α increased due to the ability of NF-κB to directly stimulate IκB-α expression (27). TPA-induced degradation of IκB-α was preceded by the appearance of a phosphorylated low-mobility isoform of IκB-α, detected 10–15 min after induction (Fig. 3 A and B). PGA1 completely prevented NF-κB activation in TPA-treated cells, while it induced HSF (Fig. 3 A and D). Treatment with deoxycholate, which disrupts the association of NF-κB with IκB, allowing the detection of inactive NF-κB, restored NF-κB binding activity in PGA1-treated samples, indicating that NF-κB was not degraded and was present in an inactive form in PGA1-treated cells (data not shown). On the other hand, the TPA-induced decrease in the IκB-α level was completely prevented in PGA1-treated cells (Fig. 3A Lower), indicating that PGA1 was interfering with a signal transduction component essential for IκB-α degradation. The fact that PGA1 prevented the appearance of the phosphorylated IκB-α isoform identifies phosphorylation as the possible target for PGA1 activity (Fig. 3B). Although it inhibited IκB-α phosphorylation, PGA1 induced HSF1 phosphorylation in Jurkat cells (Fig. 3D Lower). Whereas modulation of protein kinases’ activity by AA and several AA metabolites, including PGE2 and leukotriene B2, has been reported (30), the ability of cyclopentenone PGs to inhibit kinase activity is a novel finding.
Figure 3.
PGA1 inhibits IκB-α phosphorylation and degradation while it induces HSF activation. Jurkat cells treated with 24 μM PGA1 for 2 h in RPMI 1640 medium containing 10% FCS were stimulated with TPA for the indicated periods of time (3–90 min). Whole-cell extracts from control and TPA-treated cells were assayed for NF-κB (A Upper) or HSF (D Upper) activity by EMSA. Sections of fluorograms from native gels are shown. Positions of NF-κB and HSF are indicated as in the legend to Fig. 1. Aliquots of cell extracts from the same samples were assayed for IκB-α (A Lower) or HSF1 (D Lower) by Western blot analysis. A section of Western blot is shown. The position of IκB-α (IκBα) and the low-mobility phosphorylated isoform of IκB-α (○P-IκBα) bands are indicated in A. The position of the two higher molecular mass phosphorylated forms of HSF1, which have been described previously (23), is indicated by the arrows in D. (B) Phosphatase treatment. Equal amounts of Jurkat whole-cell extracts prepared 10 min after TPA stimulation in the presence or absence of PGA1, were either left untreated or treated with calf intestinal phosphatase (CIP) in the presence or the absence of the phosphatase inhibitor NaH2PO4. The low-mobility IκB-α isoform disappears in cell lysates treated with phosphatase. This effect is prevented by addition of the CIP inhibitor NaH2PO4 to the reaction mixture, indicating that the high molecular mass band represents a phosphorylated form of IκB-α (28). (C) Jurkat cells were pretreated with 100 μg/ml cycloheximide (CHX) for 30 min and then stimulated with TPA in the presence or absence of PGA1 (24 μM). Whole-cell extracts were prepared 6 h after TPA stimulation and analyzed for activation of NF-κB by EMSA. As reported previously (29), the nonspecific (ns) band is lacking in CHX-treated samples. This effect is not noted in the presence of PGA1.
Finally, inhibition of NF-κB was independent of de novo protein synthesis because PGA1 suppressed NF-κB activation equally in the presence or absence of CHX (Fig. 3C).
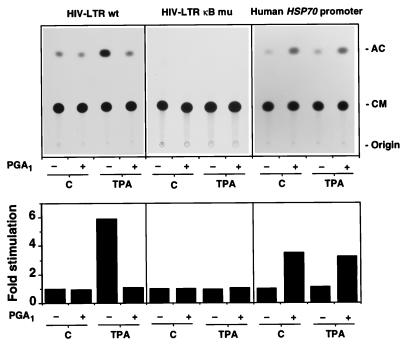
To investigate the effect of PGA1 on NF-κB and HSF-dependent transcription, Jurkat cells were transfected with a plasmid containing the HIV-1 enhancer and promoter region, which has two inducible NF-κB sites, linked to the bacterial CAT gene (HIV-LTR wt) (4) or with a construct with mutations altering the two NF-κB binding sites in the LTR (HIV-LTR κB mu) (4). Alternatively, cells were transfected with a CAT reporter gene construct containing the human HSP70 promoter sequence (LSN wt-CAT) (24). Twenty-four hours after transfection, cells were stimulated with TPA in the presence or the absence of PGA1. As expected, TPA treatment augmented CAT expression in HIV-LTR wt-transfected cells, but not in cells transfected with the mutant HIV-LTR-CAT plasmid or with the LSN wt-CAT constructs (Fig. 4). PGA1 treatment effectively inhibited the TPA-induced expression of reporter genes controlled by κB sites, while it stimulated the expression of reporter genes containing the human HSP70 promoter (Fig. 4). These results suggest that inhibition of NF-κB activation could be responsible for the recently described block of HIV-1 mRNA transcription by cyclopentenone PGs (13).
Figure 4.
Effect of PGA1 on NF-κB- and HSF-dependent transcription. Jurkat cells were transfected with a CAT reporter gene construct controlled by the promoter/enhancer of HIV-1 (HIV-LTR wt) or a construct with mutations altering the two NF-κB binding sites in the LTR (HIV-LTR κB mu). Alternatively, cells were transfected with a CAT reporter gene construct containing the human HSP70 promoter sequence (LSN wt). After 24 h cells were divided into four aliquots and stimulated with 25 ng/ml TPA for 9 h in RPMI 1640 medium supplemented with 10% FCS in the presence or absence of 24 μM PGA1: unstimulated (C) untreated (−) or PGA1-treated (+); TPA-stimulated (TPA) untreated (−) or PGA1-treated (+). The acetylated form of [14C]-1-deoxychloramphenicol (AC) was separated from unreacted reagent (CM) by ascending thin layer chromatography. An autoradiography is shown (Upper). Quantification of CAT activity is shown (Lower) and expressed as fold induction of untreated, unstimulated control. Results from three independent experiments are shown as average values.
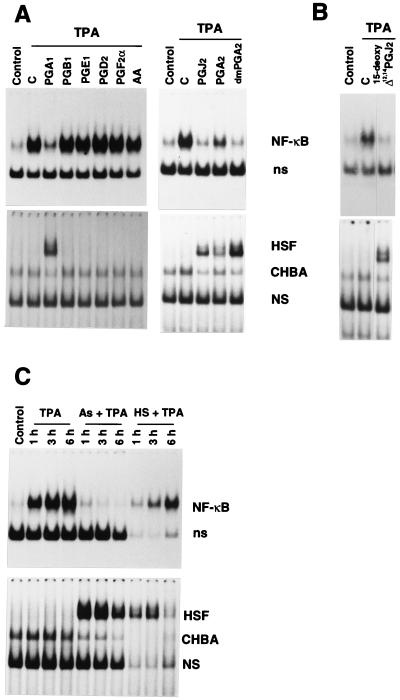
The observation that inhibition of NF-κB by PGA1 was associated with HSF activation prompted us to examine the effect of different AA metabolites, including noncyclopentenone PGs (PGE1, PGD2, PGF2α), the nonreactive cyclopentenone PGB1, reactive cyclopentenone PGs (PGA1, PGA2 16–16 dimethyl-PGA2, PGJ2, 15-deoxy-Δ12,14-PGJ2), or classic HSF inducers (sodium arsenite and heat shock) on NF-κB activation. A- and J-type PGs suppressed TPA-induced NF-κB activation while inducing HSF. AA, PGE1, PGD2, PGF2α, and the nonreactive cyclopentenone PGB1 did not activate HSF at the concentration tested and had no effect on TPA-induced NF-κB activation, indicating that the presence of a reactive cyclopentenonic moiety is essential for NF-κB inhibition (Fig. 5A). The PGJ2 metabolite 15-deoxy-Δ12,14-PGJ2, which was recently found to promote adipocyte differentiation by binding to the adipocyte determination factor PPARγ at 5 μM (31, 32), was also effective in inhibiting NF-κB while inducing HSF at the same concentration (Fig. 5B).
Figure 5.
Effect of AA metabolites and classic HSF inducers on NF-κB activation. (A) Jurkat cells were treated with 24 μM AA, PGA1, PGA2, 16,16-dimethyl-PGA2-methylester (dmPGA2), PGB1, PGD2, PGE1, PGF2α, PGJ2 (Cayman Chemicals) or diluent control (C) for 2 h and then were stimulated with TPA. Control cells were neither stimulated with TPA nor treated with PGs. Whole-cell extracts were prepared 6 h after TPA addition and subjected to EMSA for NF-κB (Upper) or HSF (Lower) activation. Positions of NF-κB and HSF are indicated as in the legend to Fig. 1. (B) Jurkat cells treated for 2 h with 5 μM 15-deoxy-Δ12,14-PGJ2 or control diluent (C) were stimulated with TPA for 3 h, and whole-cell extracts were subjected to EMSA for NF-κB (Upper) or HSF (Lower) activation. (C) Jurkat cells were either treated with 80 μM sodium arsenite (As) (Sigma) or subjected to heat shock at 45°C for 20 min (HS). Two hours after sodium arsenite treatment or heat shock, cells were stimulated with TPA. Control cells were neither stimulated with TPA nor treated with sodium arsenite or heat shock. Whole-cell extracts were prepared at 1, 3, or 6 h after TPA addition and subjected to EMSA for determination of NF-κB (Upper) or HSF (Lower) activation. In the absence of TPA, sodium arsenite and heat shock had no effect on NF-κB activation (data not shown).
Both sodium arsenite and heat shock also inhibited NF-κB activation. Interestingly, in heat-shocked cells, inhibition was reversed 6 h after TPA treatment concomitantly with the return of HSF to the inactive state, indicating that inhibition was not due to NF-κB degradation and, as for PGA1-treatment, persisted as long as HSF was activated (Fig. 5C). The possibility then exists that NF-κB could be affected by the state of transcriptional activation of HSF, or that simultaneous activation of these transcription factors could be incompatible. Inhibition of NF-κB by aspirin is also associated with HSF activation (33), an effect probably unrelated to the endogenous synthesis of eicosanoids (34).
PGs control cell proliferation and differentiation (16, 35), suppress cytokine production (7, 36), and inhibit virus replication (11). Because NF-κB activates immunoregulatory and viral genes (3, 4), inhibition of NF-κB could be one of the mechanisms involved in the immunosuppressive and antiviral activity of PGs. Inhibition of NF-κB activation and of the expression of κB-controlled HIV-LTR reporter genes is particularly relevant in view of the recently described block of HIV-1 mRNA transcription by cyclopentenone PGs, which results in a dramatic inhibition of HIV replication in acutely infected cells (13).
Cyclopentenone PGs are physiologically present in body fluids at picomolar and nanomolar concentrations (37). In several pathological conditions, including hyperthermia, infection, and inflammation (38, 39), AA metabolism is known to be highly increased; local PG concentrations in the micromolar range were detected in acute inflammation sites (40), suggesting the possibility that inhibition of NF-κB by specific AA metabolites could occur in vivo. Even though this possibility cannot be ruled out, the inhibition of NF-κB activation by micromolar concentrations of PGAs and PGJs should be viewed as a pharmacological effect, until the presence of highly elevated levels of these molecules is shown in vivo.
Finally, the finding that inhibition of NF-κB activation is a characteristic common to several HSF inducers, including hyperthermia itself, suggests a link between the regulatory pathways of these two important transcription factors and opens new perspectives in the search for effective NF-κB inhibitors as useful immunosuppressive, antiinflammatory, and antiviral drugs.
Acknowledgments
We thank Rick Morimoto for polyclonal anti-HSF1 antibodies and for LSN wt-CAT construct. This work was supported by grants from the Italian Ministry of Public Health, Ninth AIDS Research Project, and the Italian National Research Council, Progetto Finalizzato, Applicazioni Cliniche della Ricerca Oncologica, and Progetto Strategico, Stress Proteins.
Footnotes
Abbreviations: NF-κB, nuclear factor-κB; PG, prostaglandin; HSF, heat shock transcription factor; HSE, heat shock element; LTR, long terminal repeat; FCS, fetal calf serum; TNF-α, tumor necrosis factor α; EMSA, electrophoretic mobility-shift assay; CAT, chloramphenicol acetyltransferase; AA, arachidonic acid; wt, wild type; mu, mutant.
References
- 1.Thanos D, Maniatis T. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Lenardo M J, Baltimore D. Cell. 1989;58:227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- 4.Nabel G, Baltimore D. Nature (London) 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 5.Samuelsson B. In: Prostaglandins and Cancer. Powles T J, Bockman R S, Honn K V, Ramwell P, editors. New York: Liss; 1982. pp. 1–19. [Google Scholar]
- 6.Vane J. In: Prostaglandins in Cancer Research. Garaci E, Paoletti R, Santoro M G, editors. Berlin: Springer; 1987. pp. 12–28. [Google Scholar]
- 7.Ninneman J L. Prostaglandins, Leukotrienes and the Immune Response. Cambridge, U.K.: Cambridge Univ. Press; 1988. [Google Scholar]
- 8.Dinarello C A, Wolff S M. Am J Med. 1982;72:799–819. doi: 10.1016/0002-9343(82)90548-4. [DOI] [PubMed] [Google Scholar]
- 9.Robert A. In: Physiology of the Gastrointestinal Tract. Johnson L R, editor. New York: Raven; 1981. pp. 1407–1434. [Google Scholar]
- 10.Garaci E, Paoletti R, Santoro M G, editors. Prostaglandins in Cancer Research. Berlin: Springer; 1987. [Google Scholar]
- 11.Santoro M G. Experientia. 1994;50:1039–1047. doi: 10.1007/BF01923459. [DOI] [PubMed] [Google Scholar]
- 12.Santoro M G, Benedetto A, Carruba G, Garaci E, Jaffe B M. Science. 1980;209:1032–1034. doi: 10.1126/science.6157190. [DOI] [PubMed] [Google Scholar]
- 13.Rozera C, Carattoli A, De Marco A, Amici C, Giorgi C, Santoro M G. J Clin Invest. 1996;97:1795–1803. doi: 10.1172/JCI118609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amici C, Giorgi C, Rossi A, Santoro M G. J Virol. 1994;68:6890–6899. doi: 10.1128/jvi.68.11.6890-6899.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoro M G. In: Stress-Inducible Cellular Responses. Feige U, Morimoto R I, Yahara I, Polla B S, editors. Basel: Birkhäuser; 1996. pp. 335–355. [Google Scholar]
- 16.Santoro M G, Garaci E, Amici C. Proc Natl Acad Sci USA. 1989;86:8407–8411. doi: 10.1073/pnas.86.21.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amici C, Sistonen L, Santoro M G, Morimoto R I. Proc Natl Acad Sci USA. 1992;89:6227–6231. doi: 10.1073/pnas.89.14.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotto J J, Kline M, Morimoto R I. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- 19.Baeuerle P A, Baltimore D. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber E, Matthias P, Müller M M, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zabel U, Schreck R, Baeuerle P A. J Biol Chem. 1991;266:252–260. [PubMed] [Google Scholar]
- 22.Mosser D D, Theodorakis N G, Morimoto R I. Mol Cell Biol. 1988;8:4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elia G, De Marco A, Rossi A, Santoro M G. J Biol Chem. 1996;271:16111–16118. doi: 10.1074/jbc.271.27.16111. [DOI] [PubMed] [Google Scholar]
- 24.Williams G T, Morimoto R I. Mol Cell Biol. 1990;10:3125–3136. doi: 10.1128/mcb.10.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Queen C, Baltimore D. Cell. 1983;33:741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- 26.Gorman C M, Moffat L F, Howard B H. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henkel T, Machleidt T, Alkalay I, Krönke M, Ben-Neriah Y, Baeuerle P A. Nature (London) 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 28.Sun S, Ganchi P A, Béraud C, Ballard D W, Greene W C. Proc Natl Acad Sci USA. 1994;91:1346–1350. doi: 10.1073/pnas.91.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreck R, Rieber P, Baeuerle P A. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danesch U, Weber P C, Sellmayer A. J Biol Chem. 1994;269:27258–27263. [PubMed] [Google Scholar]
- 31.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 32.Kliewer S A, Lenhard J M, Willson T M, Patel I, Morris D C, Lehmann J M. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 33.Kopp E, Ghosh S. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 34.Amici C, Rossi A, Santoro M G. Cancer Res. 1995;55:4452–4457. [PubMed] [Google Scholar]
- 35.Santoro M G, Philpott G W, Jaffe B M. Nature (London) 1976;263:777–779. doi: 10.1038/263777a0. [DOI] [PubMed] [Google Scholar]
- 36.Rappaport R S, Dodge G R. J Exp Med. 1982;155:943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukushima M. Eicosanoids. 1990;3:189–199. [PubMed] [Google Scholar]
- 38.Calderwood S K, Bornstein B, Farnum E K, Stevenson M A. J Cell Physiol. 1989;141:325–333. doi: 10.1002/jcp.1041410214. [DOI] [PubMed] [Google Scholar]
- 39.Leung D Y, Key L, Steinberg J J, Young M C, Von Deck M, Wilkinson R, Geha R S. J Immunol. 1988;140:84–88. [PubMed] [Google Scholar]
- 40.Offenbacher S, Odle B M, Van Dyke T E. J Periodont Res. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]