Abstract
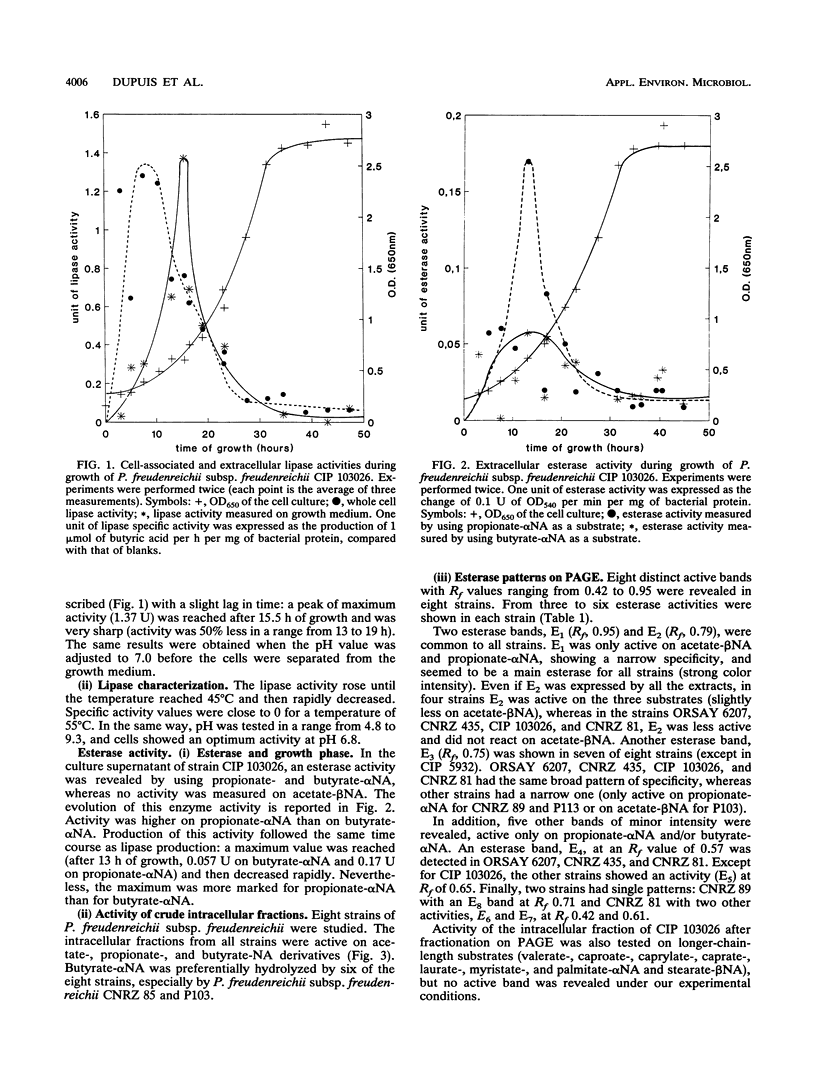
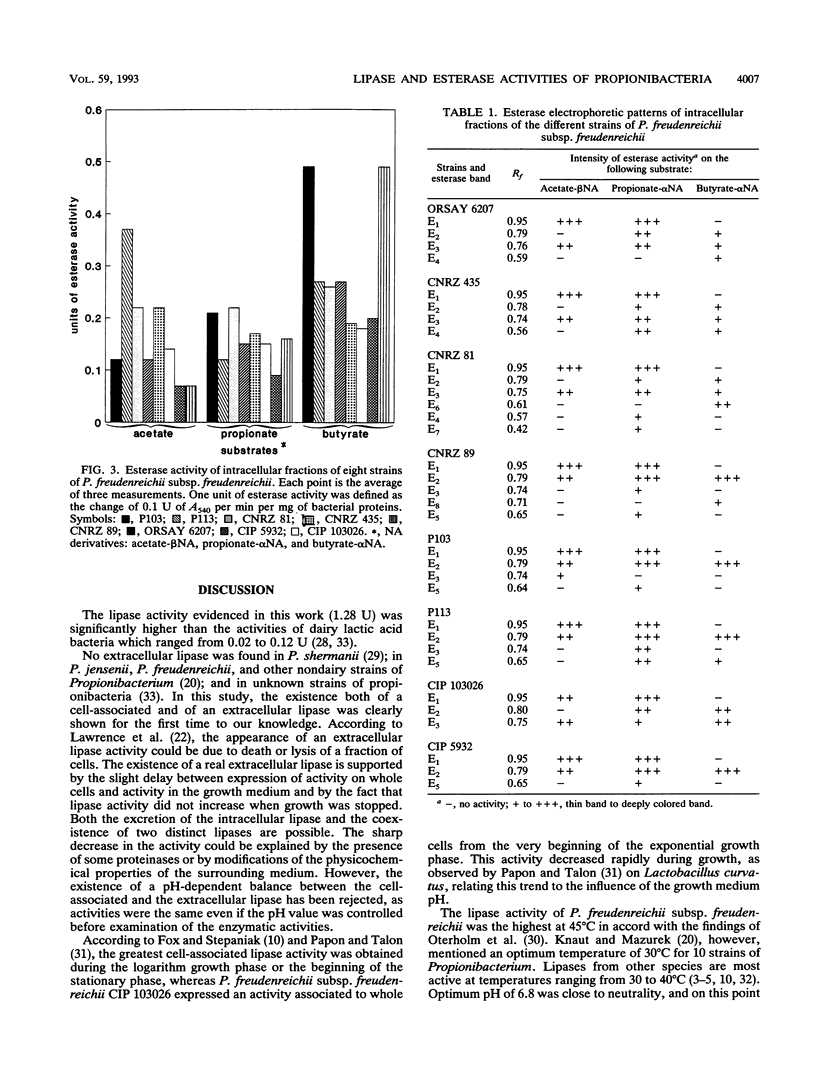
The lipase and esterase activities of eight strains of dairy Propionibacterium freudenreichii subsp. freudenreichii were studied. A lipase activity was detected on whole cells and in the culture supernatant. The highest activity was expressed at 45°C and pH 6.8. An esterase activity was also detected in the culture medium. The electrophoresis of the intracellular fractions of the cells revealed from three to six different esterase activities. Two esterases were common to all the strains. The substrate specificity was dependent on each esterase, but no activity was revealed, in our experimental conditions, on ester substrates with a chain length longer than that of butyrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castberg H. B., Solberg P. Tributyrate as a substrate for the determination of lipase activity in milk. J Dairy Res. 1975 Jun;42(2):247–253. doi: 10.1017/s0022029900015284. [DOI] [PubMed] [Google Scholar]
- Chander H., Chebbi N. B., Ranganathan B. Lipase activity of Lactobacillus brevis. Arch Mikrobiol. 1973;92(2):171–174. doi: 10.1007/BF00425014. [DOI] [PubMed] [Google Scholar]
- Collins-Thompson D. L., Sørhaug T., Witter L. D., Ordal Z. J. Glycerol Ester Hydrolase Activity of Microbacterium thermosphactum. Appl Microbiol. 1971 Jan;21(1):9–12. doi: 10.1128/am.21.1.9-12.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. F., Stepaniak L. Isolation and some properties of extracellular heat-stable lipases from Pseudomonas fluorescens strain AFT 36. J Dairy Res. 1983 Feb;50(1):77–89. doi: 10.1017/s0022029900032544. [DOI] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goullet P. Relationships between electrophoretic patterns of esterases from Salmonella. J Gen Microbiol. 1977 Feb;98(2):535–542. doi: 10.1099/00221287-98-2-535. [DOI] [PubMed] [Google Scholar]
- Guillou J. P., Chevrier L. Etude et différenciation de certaines hydrolases actives sur les triglycérides et les esters, chez les bactéries anaérobies, par la méthode de chromatographie en gaz-liquide. Ann Microbiol (Paris) 1979 Nov-Dec;130B(4):399–406. [PubMed] [Google Scholar]
- Holland K. T., Greenman J., Cunliffe W. J. Growth of cutaneous propionibacteria on synthetic medium; growth yields and exoenzyme production. J Appl Bacteriol. 1979 Dec;47(3):383–394. doi: 10.1111/j.1365-2672.1979.tb01198.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence R. C., Fryer T. F., Reiter B. The production and characterization of lipases from a micrococcus and a pseudomonad. J Gen Microbiol. 1967 Sep;48(3):401–418. doi: 10.1099/00221287-48-3-401. [DOI] [PubMed] [Google Scholar]
- Lund B. M. A comparison by the use of gel electrophoresis of soluble protein components and esterase enzymes of some group D Streptococci. J Gen Microbiol. 1965 Sep;40(3):413–419. doi: 10.1099/00221287-40-3-413. [DOI] [PubMed] [Google Scholar]
- Malik A. C., Reinbold G. W., Vedamuthu E. R. An evaluation of the taxonomy of Propionibacterium. Can J Microbiol. 1968 Nov;14(11):1185–1191. doi: 10.1139/m68-199. [DOI] [PubMed] [Google Scholar]
- Morichi T., Sharpe M. E., Reiter B. Esterases and other soluble proteins of some lactic acid bacteria. J Gen Microbiol. 1968 Oct;53(3):405–414. doi: 10.1099/00221287-53-3-405. [DOI] [PubMed] [Google Scholar]
- Oterholm A., Ordal Z. J., Witter L. D. Glycerol ester hydrolase activity of lactic acid bacteria. Appl Microbiol. 1968 Mar;16(3):524–527. doi: 10.1128/am.16.3.524-527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oterholm A., Ordal Z. J., Witter L. D. Purification and properties of a glycerol ester hydrolase (lipase) from Propionibacterium shermanii. Appl Microbiol. 1970 Jul;20(1):16–22. doi: 10.1128/am.20.1.16-22.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papon M., Talon R. Factors affecting growth and lipase production by meat lactobacilli strains and Brochothrix thermosphacta. J Appl Bacteriol. 1988 Feb;64(2):107–115. doi: 10.1111/j.1365-2672.1988.tb02729.x. [DOI] [PubMed] [Google Scholar]
- Unkles S. E., Gemmell C. G. Effect of clindamycin, erythromycin, lincomycin, and tetracycline on growth and extracellular lipase production by propionibacteria in vitro. Antimicrob Agents Chemother. 1982 Jan;21(1):39–43. doi: 10.1128/aac.21.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


