Abstract
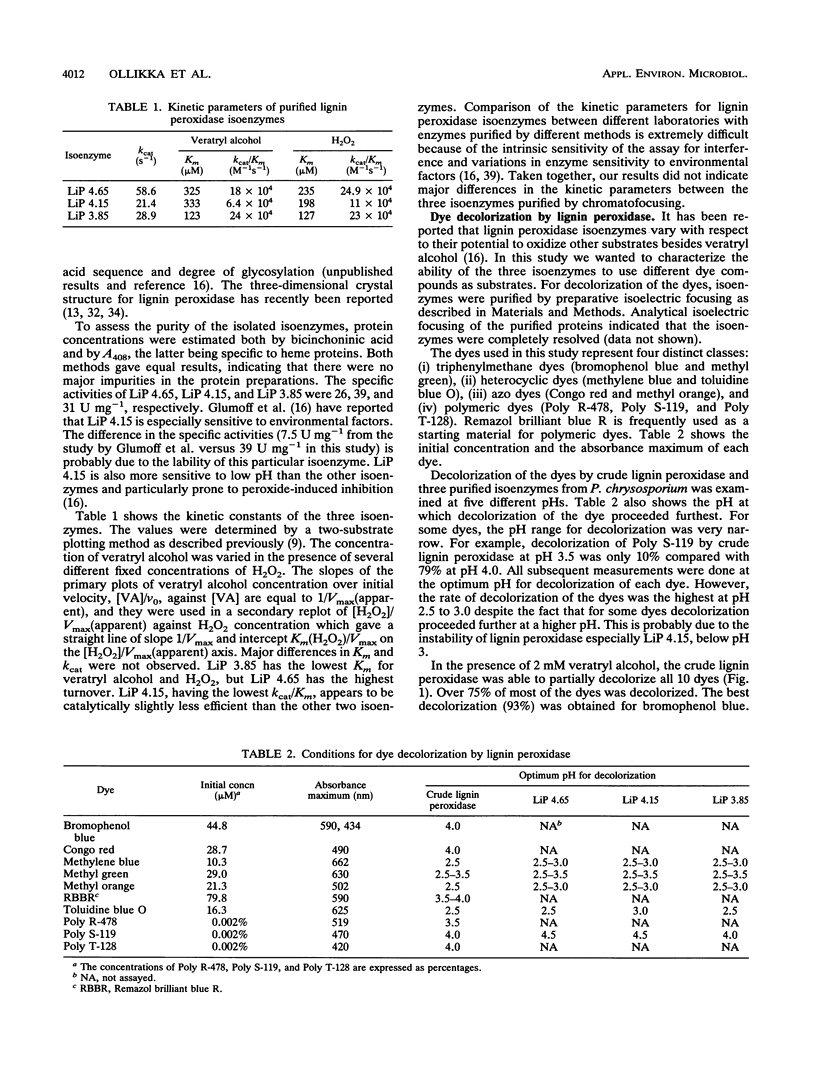
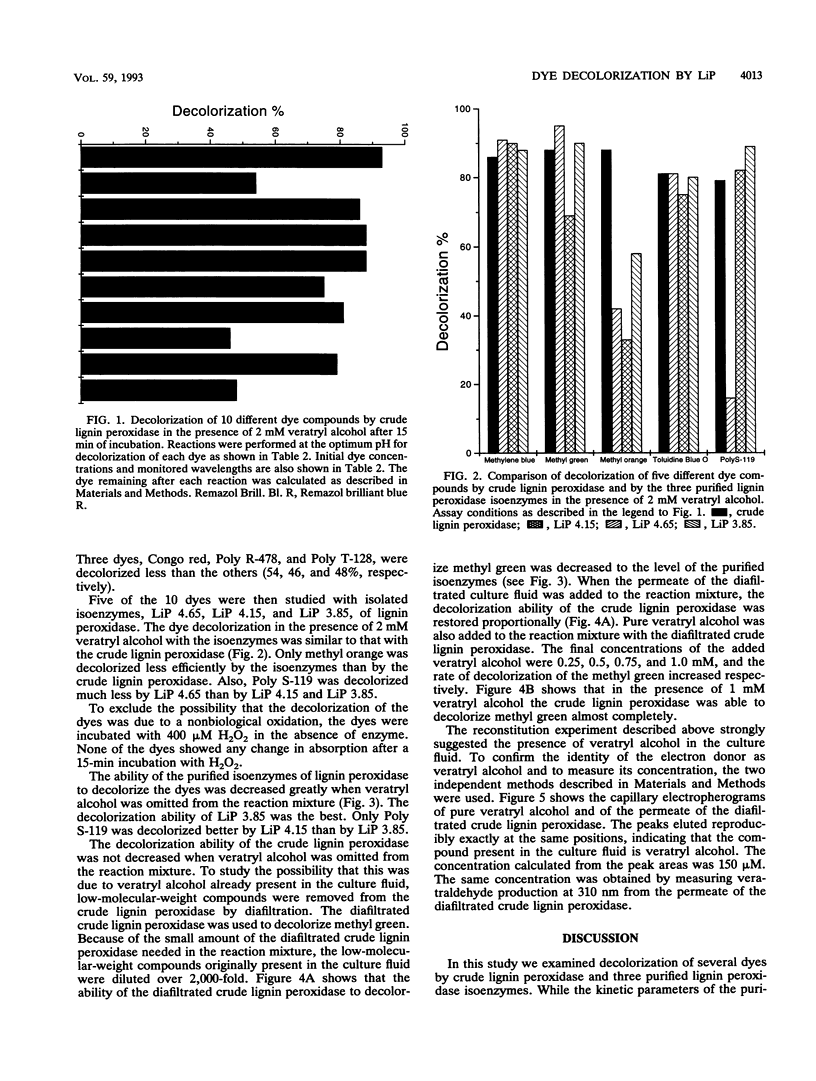
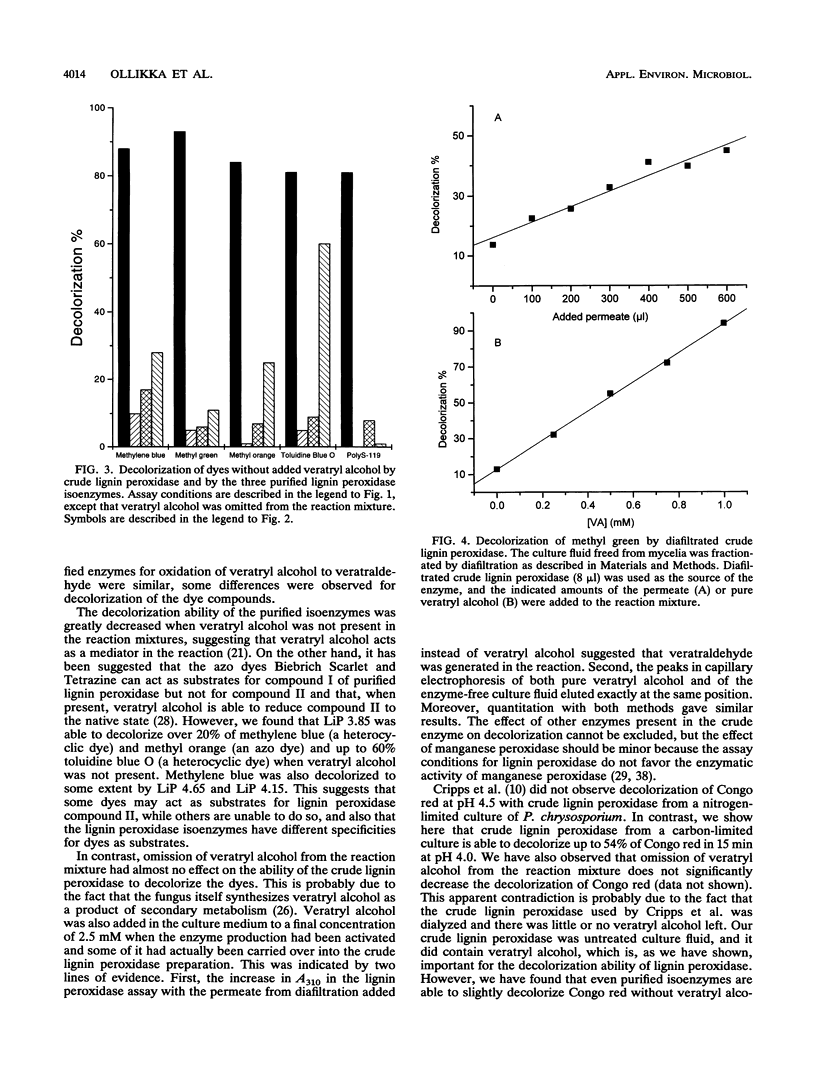
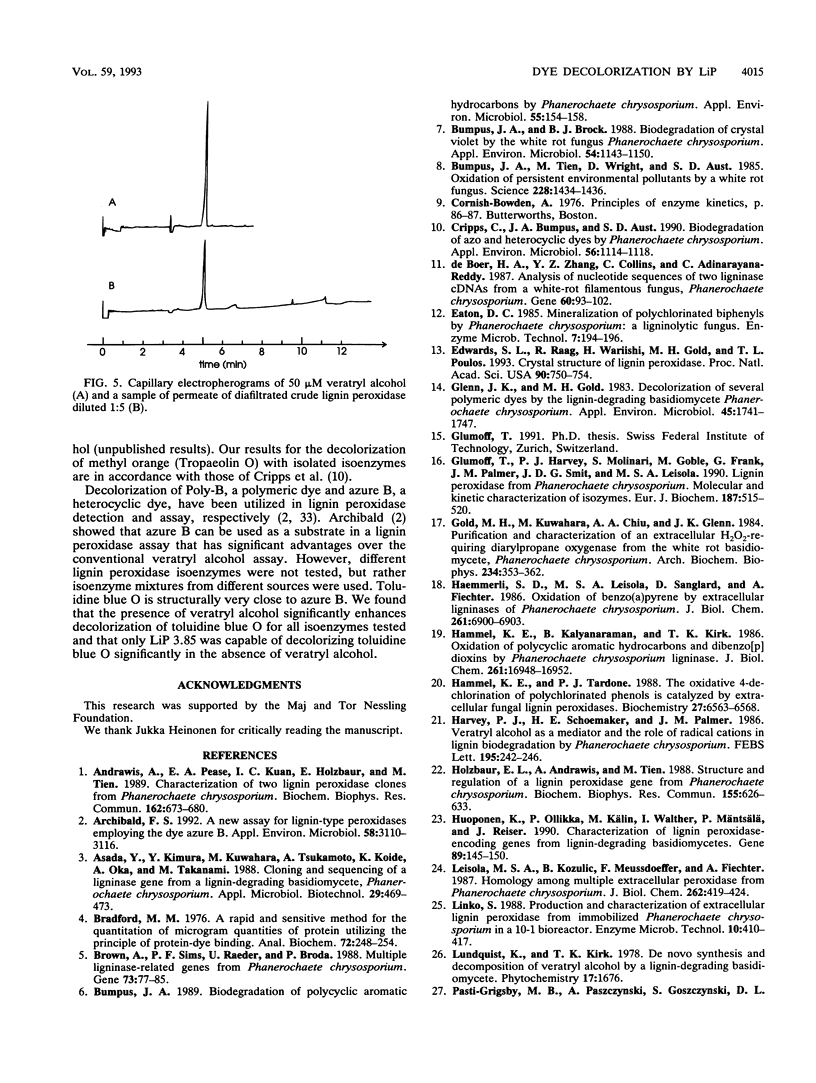
The ligninolytic enzyme system of Phanerochaete chrysosporium decolorizes several recalcitrant dyes. Three isolated lignin peroxidase isoenzymes (LiP 4.65, LiP 4.15, and LiP 3.85) were compared as decolorizers with the crude enzyme system from the culture medium. LiP 4.65 (H2), LiP 4.15 (H7), and LiP 3.85 (H8) were purified by chromatofocusing, and their kinetic parameters were found to be similar. Ten different types of dyes, including azo, triphenyl methane, heterocyclic, and polymeric dyes, were treated by the crude enzyme preparation. Most of the dyes lost over 75% of their color; only Congo red, Poly R-478, and Poly T-128 were decolorized less than the others, 54, 46, and 48%, respectively. Five different dyes were tested for decolorization by the three purified isoenzymes. The ability of the isoenzymes to decolorize the dyes in the presence of veratryl alcohol was generally comparable to that of the crude enzyme preparation, suggesting that lignin peroxidase plays a major role in the decolorization and that manganese peroxidase is not required to start the degradation of these dyes. In the absence of veratryl alcohol, the decolorization activity of the isoenzymes was in most cases dramatically reduced. However, LiP 3.85 was still able to decolorize 20% of methylene blue and methyl orange and as much as 60% of toluidine blue O, suggesting that at least some dyes can function as substrates for isoenzyme LiP 3.85 but not to the same extent for LiP 4.15 or LiP 4.65. Thus, the isoenzymes have different specificities towards dyes as substrates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrawis A., Pease E. A., Kuan I. C., Holzbaur E., Tien M. Characterization of two lignin peroxidase clones from Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1989 Jul 31;162(2):673–680. doi: 10.1016/0006-291x(89)92363-2. [DOI] [PubMed] [Google Scholar]
- Archibald F. S. A new assay for lignin-type peroxidases employing the dye azure B. Appl Environ Microbiol. 1992 Sep;58(9):3110–3116. doi: 10.1128/aem.58.9.3110-3116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown A., Sims P. F., Raeder U., Broda P. Multiple ligninase-related genes from Phanerochaete chrysosporium. Gene. 1988 Dec 15;73(1):77–85. doi: 10.1016/0378-1119(88)90314-9. [DOI] [PubMed] [Google Scholar]
- Bumpus J. A. Biodegradation of polycyclic hydrocarbons by Phanerochaete chrysosporium. Appl Environ Microbiol. 1989 Jan;55(1):154–158. doi: 10.1128/aem.55.1.154-158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Brock B. J. Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 May;54(5):1143–1150. doi: 10.1128/aem.54.5.1143-1150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Cripps C., Bumpus J. A., Aust S. D. Biodegradation of azo and heterocyclic dyes by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Apr;56(4):1114–1118. doi: 10.1128/aem.56.4.1114-1118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. L., Raag R., Wariishi H., Gold M. H., Poulos T. L. Crystal structure of lignin peroxidase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):750–754. doi: 10.1073/pnas.90.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Decolorization of Several Polymeric Dyes by the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Jun;45(6):1741–1747. doi: 10.1128/aem.45.6.1741-1747.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glumoff T., Harvey P. J., Molinari S., Goble M., Frank G., Palmer J. M., Smit J. D., Leisola M. S. Lignin peroxidase from Phanerochaete chrysosporium. Molecular and kinetic characterization of isozymes. Eur J Biochem. 1990 Feb 14;187(3):515–520. doi: 10.1111/j.1432-1033.1990.tb15333.x. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Oxidation of polycyclic aromatic hydrocarbons and dibenzo[p]-dioxins by Phanerochaete chrysosporium ligninase. J Biol Chem. 1986 Dec 25;261(36):16948–16952. [PubMed] [Google Scholar]
- Holzbaur E. L., Andrawis A., Tien M. Structure and regulation of a lignin peroxidase gene from Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1988 Sep 15;155(2):626–633. doi: 10.1016/s0006-291x(88)80541-2. [DOI] [PubMed] [Google Scholar]
- Huoponen K., Ollikka P., Kälin M., Walther I., Mäntsälä P., Reiser J. Characterization of lignin peroxidase-encoding genes from lignin-degrading basidiomycetes. Gene. 1990 Apr 30;89(1):145–150. doi: 10.1016/0378-1119(90)90218-g. [DOI] [PubMed] [Google Scholar]
- Leisola M. S., Kozulic B., Meussdoerffer F., Fiechter A. Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem. 1987 Jan 5;262(1):419–424. [PubMed] [Google Scholar]
- Pasti-Grigsby M. B., Paszczynski A., Goszczynski S., Crawford D. L., Crawford R. L. Influence of aromatic substitution patterns on azo dye degradability by Streptomyces spp. and Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Nov;58(11):3605–3613. doi: 10.1128/aem.58.11.3605-3613.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszczynski A., Crawford R. L. Degradation of azo compounds by ligninase from Phanerochaete chrysosporium: involvement of veratryl alcohol. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1056–1063. doi: 10.1016/0006-291x(91)90999-n. [DOI] [PubMed] [Google Scholar]
- Paszczynski A., Pasti-Grigsby M. B., Goszczynski S., Crawford R. L., Crawford D. L. Mineralization of sulfonated azo dyes and sulfanilic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl Environ Microbiol. 1992 Nov;58(11):3598–3604. doi: 10.1128/aem.58.11.3598-3604.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek K., Glumoff T., Winterhalter K. Low pH crystal structure of glycosylated lignin peroxidase from Phanerochaete chrysosporium at 2.5 A resolution. FEBS Lett. 1993 Jan 4;315(2):119–124. doi: 10.1016/0014-5793(93)81146-q. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Edwards S. L., Wariishi H., Gold M. H. Crystallographic refinement of lignin peroxidase at 2 A. J Biol Chem. 1993 Feb 25;268(6):4429–4440. doi: 10.2210/pdb1lga/pdb. [DOI] [PubMed] [Google Scholar]
- Schalch H., Gaskell J., Smith T. L., Cullen D. Molecular cloning and sequences of lignin peroxidase genes of Phanerochaete chrysosporium. Mol Cell Biol. 1989 Jun;9(6):2743–2747. doi: 10.1128/mcb.9.6.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spadaro J. T., Gold M. H., Renganathan V. Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Aug;58(8):2397–2401. doi: 10.1128/aem.58.8.2397-2401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K., Bull C., Fee J. A. Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerocheate chrysosporium Burds. J Biol Chem. 1986 Feb 5;261(4):1687–1693. [PubMed] [Google Scholar]
- Tien M., Tu C. P. Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature. 1987 Apr 2;326(6112):520–523. doi: 10.1038/326520a0. [DOI] [PubMed] [Google Scholar]
- Walther I., Kälin M., Reiser J., Suter F., Fritsche B., Saloheimo M., Leisola M., Teeri T., Knowles J. K., Fiechter A. Molecular analysis of a Phanerochaete chrysosporium lignin peroxidase gene. Gene. 1988 Oct 15;70(1):127–137. doi: 10.1016/0378-1119(88)90111-4. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Zhang Y. Z., Collins C., Reddy C. A. Analysis of nucleotide sequences of two ligninase cDNAs from a white-rot filamentous fungus, Phanerochaete chrysosporium. Gene. 1987;60(1):93–102. doi: 10.1016/0378-1119(87)90217-4. [DOI] [PubMed] [Google Scholar]



