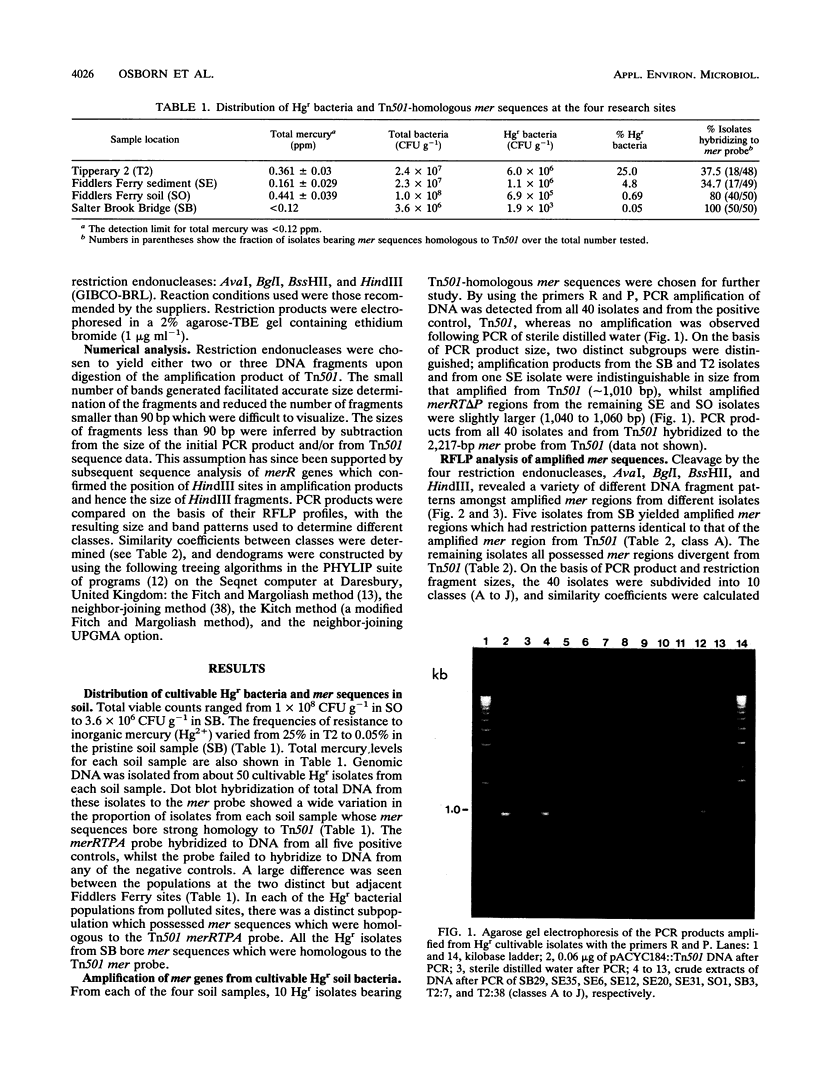
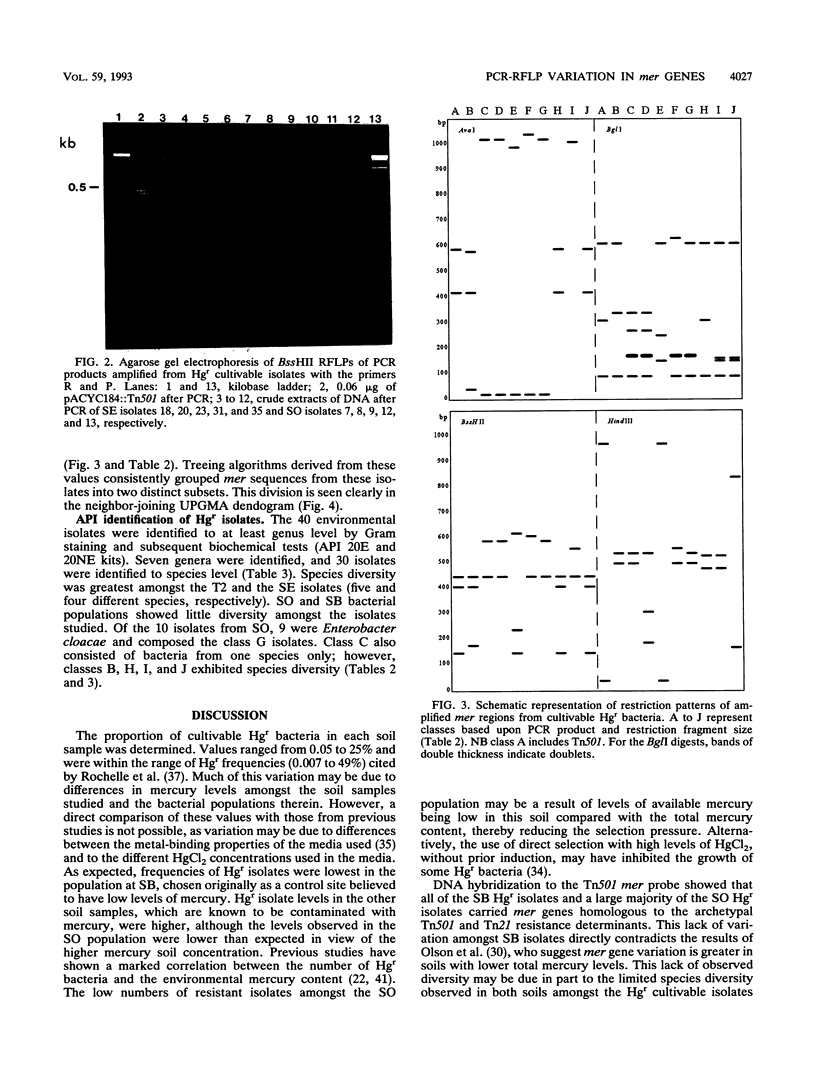
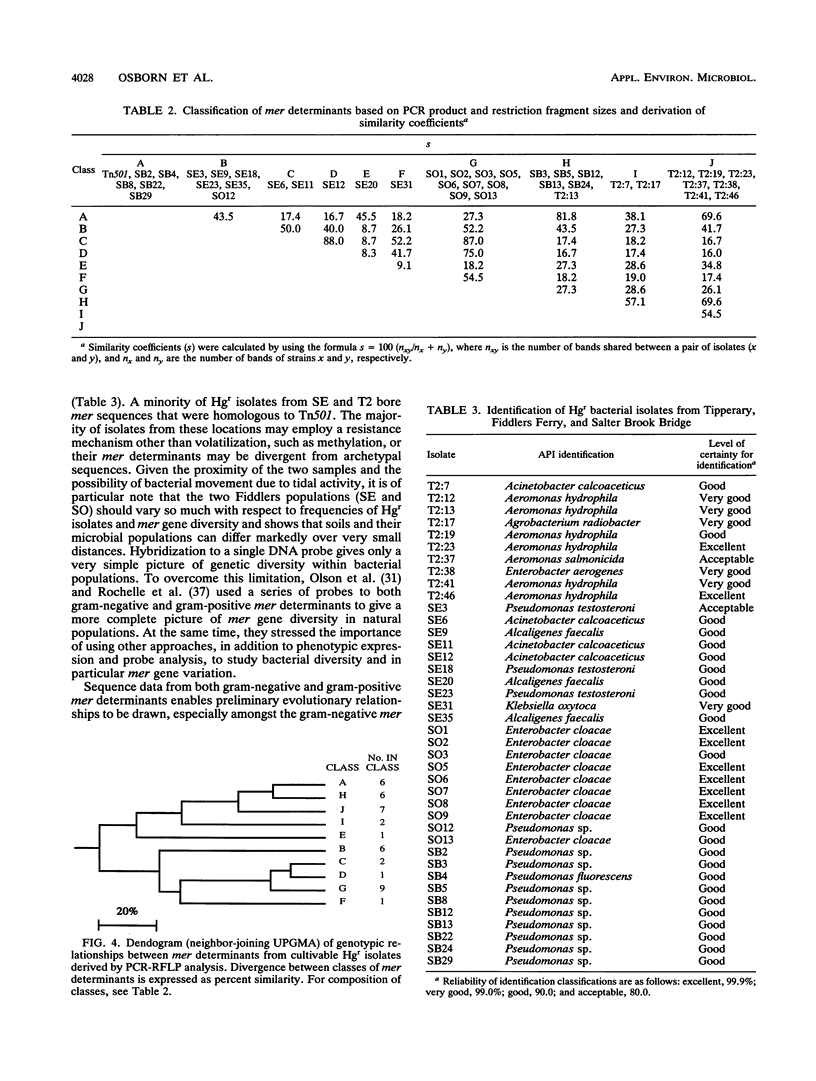
Abstract
Mercury resistant (Hgr) bacteria were isolated from four terrestrial sites: three containing high levels of mercury (sites T2, SE, and SO) and one uncontaminated site (SB). The frequencies of Hgr bacteria in the total cultivable populations were 0.05% (SB), 0.69% (SO), 4.8% (SE), and 25% (T2). Between 35 and 100% of the isolates from the four sites contained DNA sequences homologous to a DNA probe from the mercury resistance (mer) operon of the Tn501 Hgr determinant. The mer sequences of 10 Tn501-homologous Hgr determinants from each site were amplified by the polymerase chain reaction, with primers designed to consensus sequences of the mer determinants of Tn501, Tn21, and pMJ100, and were classified on the basis of the size of the amplified product and the restriction fragment length polymorphism pattern. Two main groups of amplification product were identified. The first, represented by the T2 and SB isolates and one SE isolate, gave an amplification product indistinguishable in size from that amplified from Tn501 (approximately 1,010 bp). The second group, represented by the SO isolates and the majority of the SE isolates, produced larger amplification products of 1,040 or 1,060 bp. Restriction fragment length polymorphism analysis revealed that each amplification product size group could be further subdivided into five subgroups.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkay T., Fouts D. L., Olson B. H. Preparation of a DNA gene probe for detection of mercury resistance genes in gram-negative bacterial communities. Appl Environ Microbiol. 1985 Mar;49(3):686–692. doi: 10.1128/aem.49.3.686-692.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkay T., Liebert C., Gillman M. Hybridization of DNA probes with whole-community genome for detection of genes that encode microbial responses to pollutants: mer genes and Hg2+ resistance. Appl Environ Microbiol. 1989 Jun;55(6):1574–1577. doi: 10.1128/aem.55.6.1574-1577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrineau P., Gilbert P., Jackson W. J., Jones C. S., Summers A. O., Wisdom S. The DNA sequence of the mercury resistance operon of the IncFII plasmid NR1. J Mol Appl Genet. 1984;2(6):601–619. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova E. S., Mindlin S. Z., Kalyaeva E. S., Nikiforov V. G. The diversity of mercury reductases among mercury-resistant bacteria. FEBS Lett. 1988 Jul 18;234(2):280–282. doi: 10.1016/0014-5793(88)80098-x. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Ford S. J., Pridmore R. D., Fritzinger D. C. Nucleotide sequence of a gene from the Pseudomonas transposon Tn501 encoding mercuric reductase. Biochemistry. 1983 Aug 16;22(17):4089–4095. doi: 10.1021/bi00286a015. [DOI] [PubMed] [Google Scholar]
- Bruce K. D., Hiorns W. D., Hobman J. L., Osborn A. M., Strike P., Ritchie D. A. Amplification of DNA from native populations of soil bacteria by using the polymerase chain reaction. Appl Environ Microbiol. 1992 Oct;58(10):3413–3416. doi: 10.1128/aem.58.10.3413-3416.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan G. W., Langston W. J. Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environ Pollut. 1992;76(2):89–131. doi: 10.1016/0269-7491(92)90099-v. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. E. Existence of the colicinogenic factor-sex factor ColI-b-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem Biophys Res Commun. 1970 Oct 9;41(1):150–156. doi: 10.1016/0006-291x(70)90481-x. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gilbert M. P., Summers A. O. The distribution and divergence of DNA sequences related to the Tn21 and Tn501 mer operons. Plasmid. 1988 Sep;20(2):127–136. doi: 10.1016/0147-619x(88)90015-7. [DOI] [PubMed] [Google Scholar]
- Griffin H. G., Foster T. J., Silver S., Misra T. K. Cloning and DNA sequence of the mercuric- and organomercurial-resistance determinants of plasmid pDU1358. Proc Natl Acad Sci U S A. 1987 May;84(10):3112–3116. doi: 10.1073/pnas.84.10.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling M. G., Peters S. E., Ritchie D. A. Restriction pattern and polypeptide homology among plasmid-borne mercury resistance determinants. Plasmid. 1988 Sep;20(2):106–112. doi: 10.1016/0147-619x(88)90013-3. [DOI] [PubMed] [Google Scholar]
- Khesin R. B., Karasyova E. V. Mercury-resistant plasmids in bacteria from a mercury and antimony deposit area. Mol Gen Genet. 1984;197(2):280–285. doi: 10.1007/BF00330974. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Liesack W., Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992 Aug;174(15):5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaia O. L., Nikiforov V. G. Nukleotidnye posledovatel'nosti determinant ustoichivosti k rtuti bakterii, obitaiushchikh v rtutnykh mestorozhdeniiakh: obnaruzhenie semeistv rekombinantnykh rtutnykh transpozonov v plazmidakh bakterii roda Acinetobacter. Genetika. 1988 Sep;24(9):1539–1549. [PubMed] [Google Scholar]
- Misra T. K. Bacterial resistances to inorganic mercury salts and organomercurials. Plasmid. 1992 Jan;27(1):4–16. doi: 10.1016/0147-619x(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Misra T. K., Brown N. L., Fritzinger D. C., Pridmore R. D., Barnes W. M., Haberstroh L., Silver S. Mercuric ion-resistance operons of plasmid R100 and transposon Tn501: the beginning of the operon including the regulatory region and the first two structural genes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5975–5979. doi: 10.1073/pnas.81.19.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro E., Simonet P., Normand P., Bardin R. Characterization of natural populations of Nitrobacter spp. using PCR/RFLP analysis of the ribosomal intergenic spacer. Arch Microbiol. 1992;157(2):107–115. doi: 10.1007/BF00245277. [DOI] [PubMed] [Google Scholar]
- Peters S. E., Hobman J. L., Strike P., Ritchie D. A. Novel mercury resistance determinants carried by IncJ plasmids pMERPH and R391. Mol Gen Genet. 1991 Aug;228(1-2):294–299. doi: 10.1007/BF00282479. [DOI] [PubMed] [Google Scholar]
- Radford A. J., Oliver J., Kelly W. J., Reanney D. C. Translocatable resistance to mercuric and phenylmercuric ions in soil bacteria. J Bacteriol. 1981 Sep;147(3):1110–1112. doi: 10.1128/jb.147.3.1110-1112.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle Paul A., Wetherbee Mary K., Olson Betty H. Distribution of DNA Sequences Encoding Narrow- and Broad-Spectrum Mercury Resistance. Appl Environ Microbiol. 1991 Jun;57(6):1581–1589. doi: 10.1128/aem.57.6.1581-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Silver S., Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992 Mar;56(1):195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M., Richmond M. H. Characterization of a translocation unit encoding resistance to mercuric ions that occurs on a nonconjugative plasmid in Pseudomonas aeruginosa. J Bacteriol. 1977 Mar;129(3):1227–1233. doi: 10.1128/jb.129.3.1227-1233.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoney J. F., Port J., Giles J., Spanier J. Heavy-metal and antibiotic resistance in the bacterial flora of sediments of New York Bight. Appl Environ Microbiol. 1978 Sep;36(3):465–472. doi: 10.1128/aem.36.3.465-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatcroft R., Williams P. A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981 Jun;124(2):433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]