Abstract
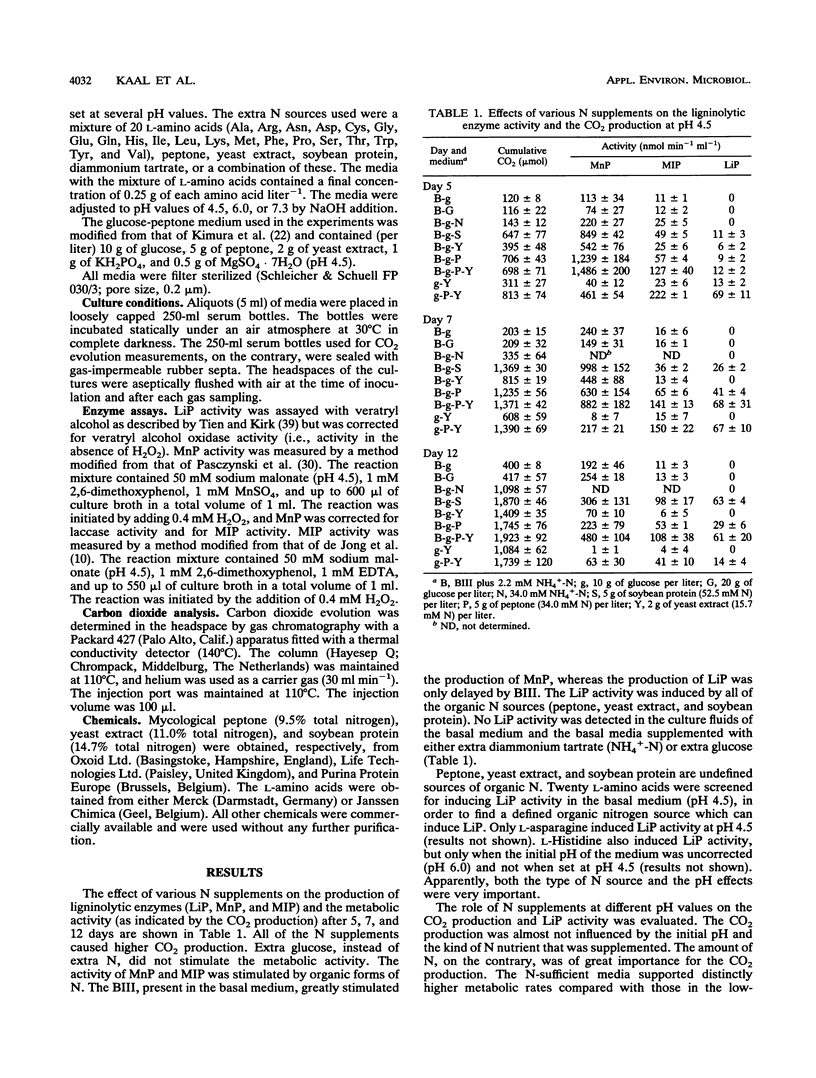
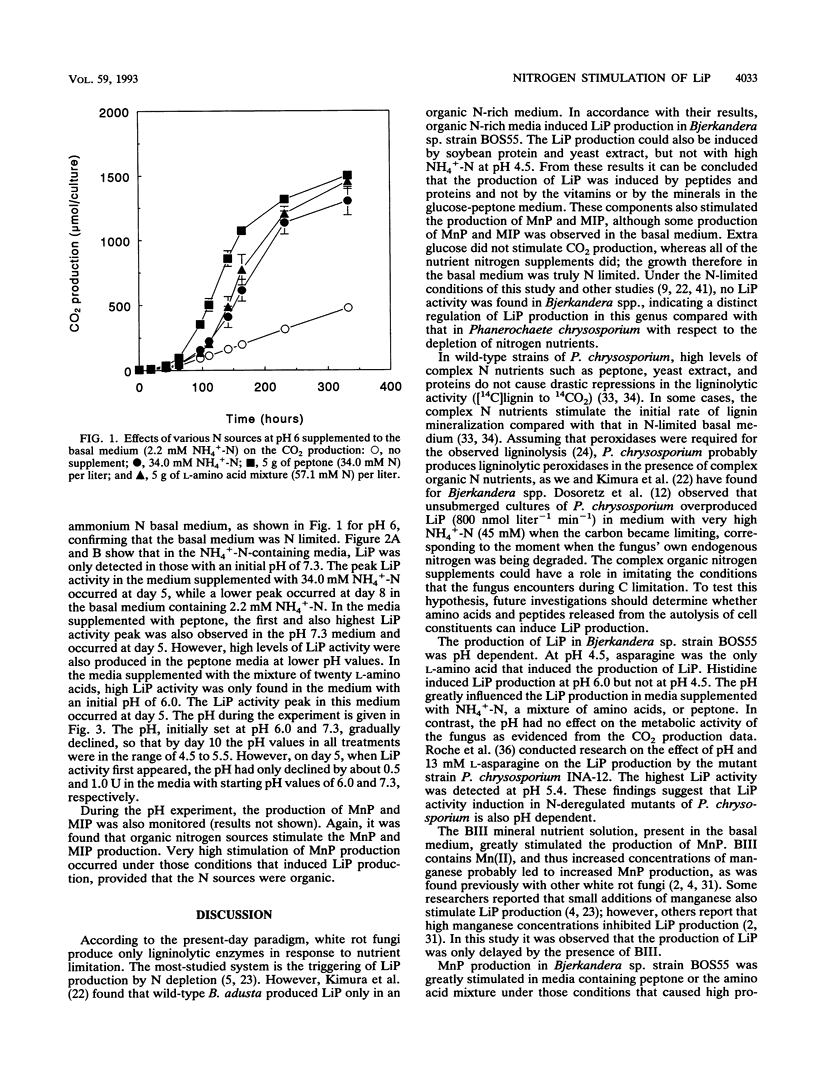
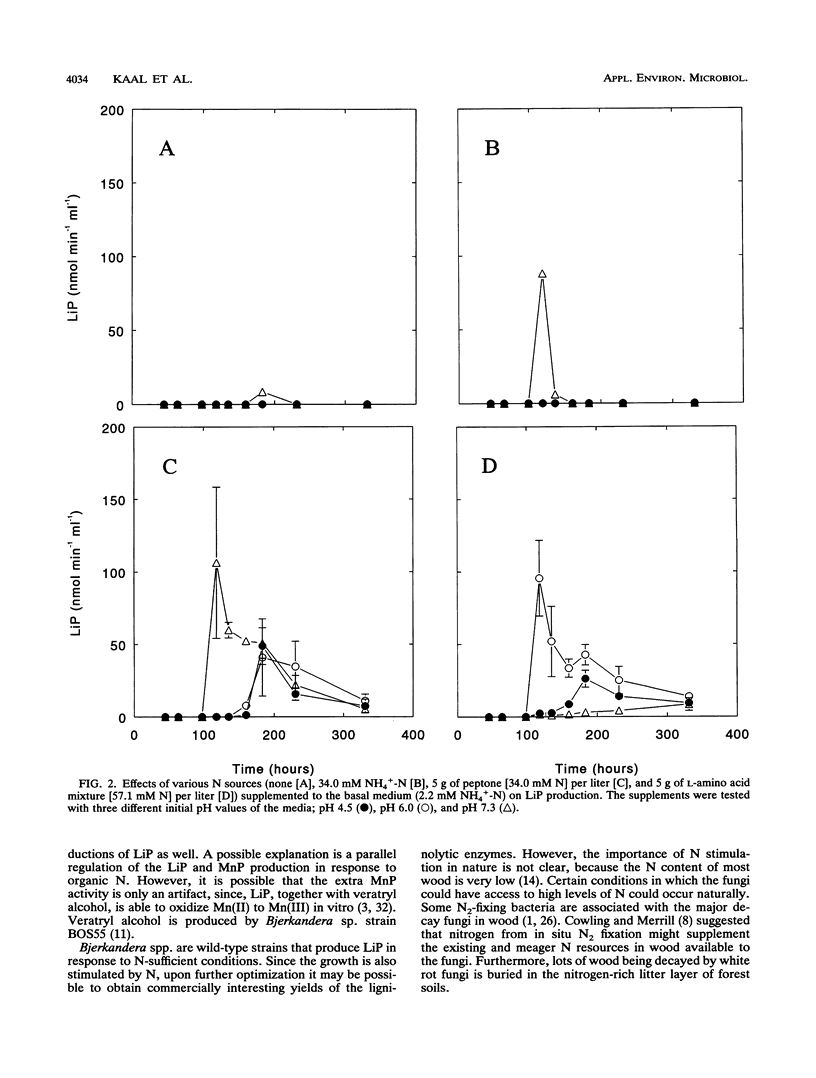
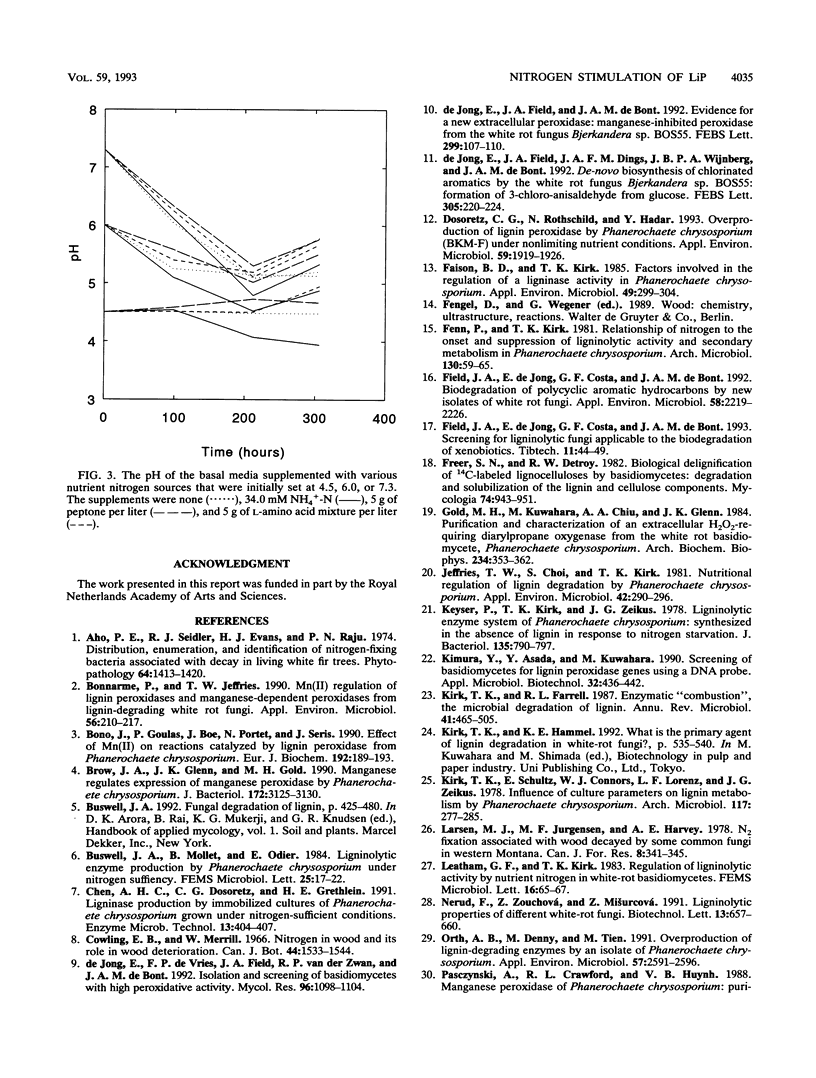
Bjerkandera sp. strain BOS55, a newly isolated wild-type white rot fungus, produced lignin peroxidase (LiP) in nitrogen (N)-sufficient glucose-peptone medium, whereas no LiP was detectable in N-limited medium. The production of LiP was induced by the peptide-containing components of this medium and also by soy bean protein. Furthermore, the production of manganese-dependent peroxidase was stimulated by organic N sources, although lower production was also evident in N-limited medium. Further research showed that the induction of LiP depended on the combination of pH and the type of N source. An amino acid mixture and ammonium induced LiP only at either pH 6 or 7.3, respectively. Peptone induced LiP activity at all pH values tested; however, the highest activity was observed at pH 7.3. The results presented here indicate that Bjerkandera spp. are distinct from the model white rot fungus, Phanerochaete chrysosporium, which produces ligninolytic peroxidases in response to N limitation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono J. J., Goulas P., Boe J. F., Portet N., Seris J. L. Effect of Mn(II) on reactions catalyzed by lignin peroxidase from Phanerochaete chrysosporium. Eur J Biochem. 1990 Aug 28;192(1):189–193. doi: 10.1111/j.1432-1033.1990.tb19213.x. [DOI] [PubMed] [Google Scholar]
- Brown J. A., Glenn J. K., Gold M. H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990 Jun;172(6):3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Rothschild N., Hadar Y. Overproduction of lignin peroxidase by Phanerochaete chrysosporium (BKM-F-1767) under nonlimiting nutrient conditions. Appl Environ Microbiol. 1993 Jun;59(6):1919–1926. doi: 10.1128/aem.59.6.1919-1926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J. A., de Jong E., Feijoo Costa G., de Bont J. A. Biodegradation of polycyclic aromatic hydrocarbons by new isolates of white rot fungi. Appl Environ Microbiol. 1992 Jul;58(7):2219–2226. doi: 10.1128/aem.58.7.2219-2226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Asada Y., Kuwahara M. Screening of basidiomycetes for lignin peroxidase genes using a DNA probe. Appl Microbiol Biotechnol. 1990 Jan;32(4):436–442. doi: 10.1007/BF00903779. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Orth A. B., Denny M., Tien M. Overproduction of lignin-degrading enzymes by an isolate of Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Sep;57(9):2591–2596. doi: 10.1128/aem.57.9.2591-2596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Aug;58(8):2402–2409. doi: 10.1128/aem.58.8.2402-2409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp J. L., Kalyanaraman B., Kirk T. K. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry. 1990 Nov 20;29(46):10475–10480. doi: 10.1021/bi00498a008. [DOI] [PubMed] [Google Scholar]
- Reid I. D. Effects of Nitrogen Sources on Cellulose and Synthetic Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Mar;45(3):838–842. doi: 10.1128/aem.45.3.838-842.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid I. D. Effects of Nitrogen Supplements on Degradation of Aspen Wood Lignin and Carbohydrate Components by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Mar;45(3):830–837. doi: 10.1128/aem.45.3.830-837.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Myer S. B. Selection and characterization of mutants of Phanerochaete chrysosporium exhibiting ligninolytic activity under nutrient-rich conditions. Appl Environ Microbiol. 1990 Aug;56(8):2540–2544. doi: 10.1128/aem.56.8.2540-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong E., Field J. A., Dings J. A., Wijnberg J. B., de Bont J. A. De-novo biosynthesis of chlorinated aromatics by the white-rot fungus Bjerkandera sp. BOS55. Formation of 3-chloro-anisaldehyde from glucose. FEBS Lett. 1992 Jul 6;305(3):220–224. doi: 10.1016/0014-5793(92)80672-4. [DOI] [PubMed] [Google Scholar]
- de Jong E., Field J. A., de Bont J. A. Evidence for a new extracellular peroxidase. Manganese-inhibited peroxidase from the white-rot fungus Bjerkandera sp. BOS 55. FEBS Lett. 1992 Mar 24;299(1):107–110. doi: 10.1016/0014-5793(92)80111-s. [DOI] [PubMed] [Google Scholar]



