Abstract
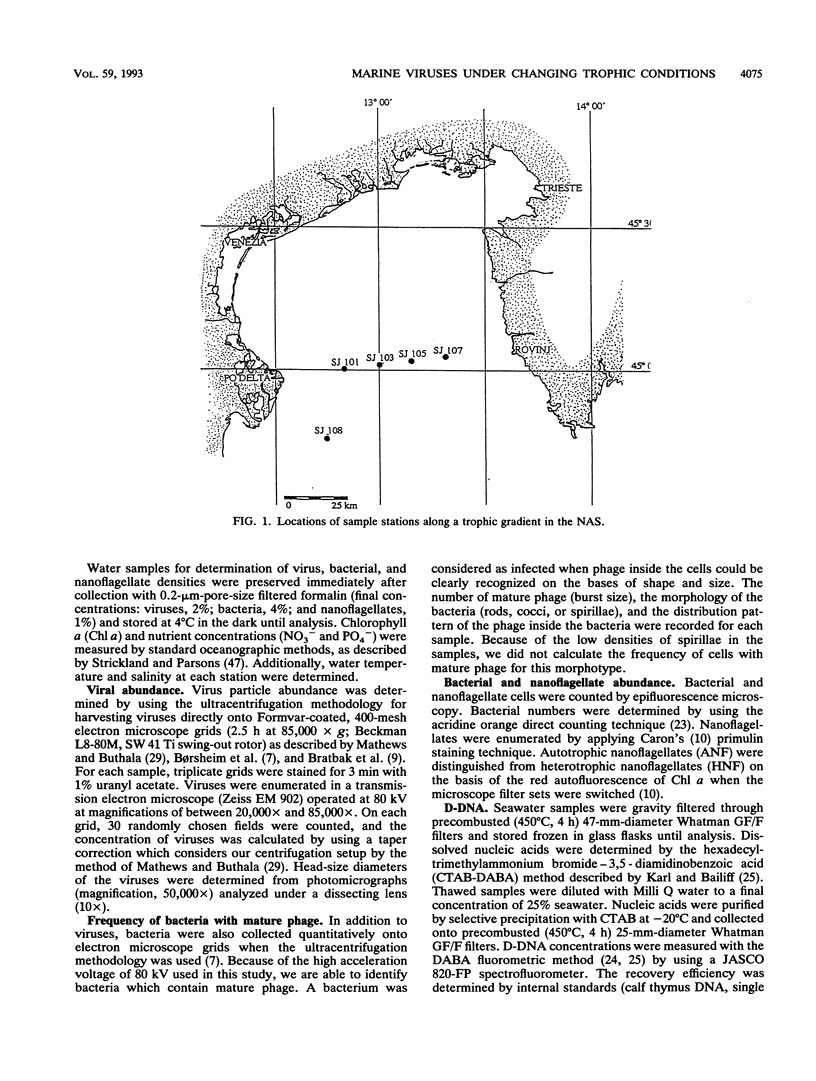
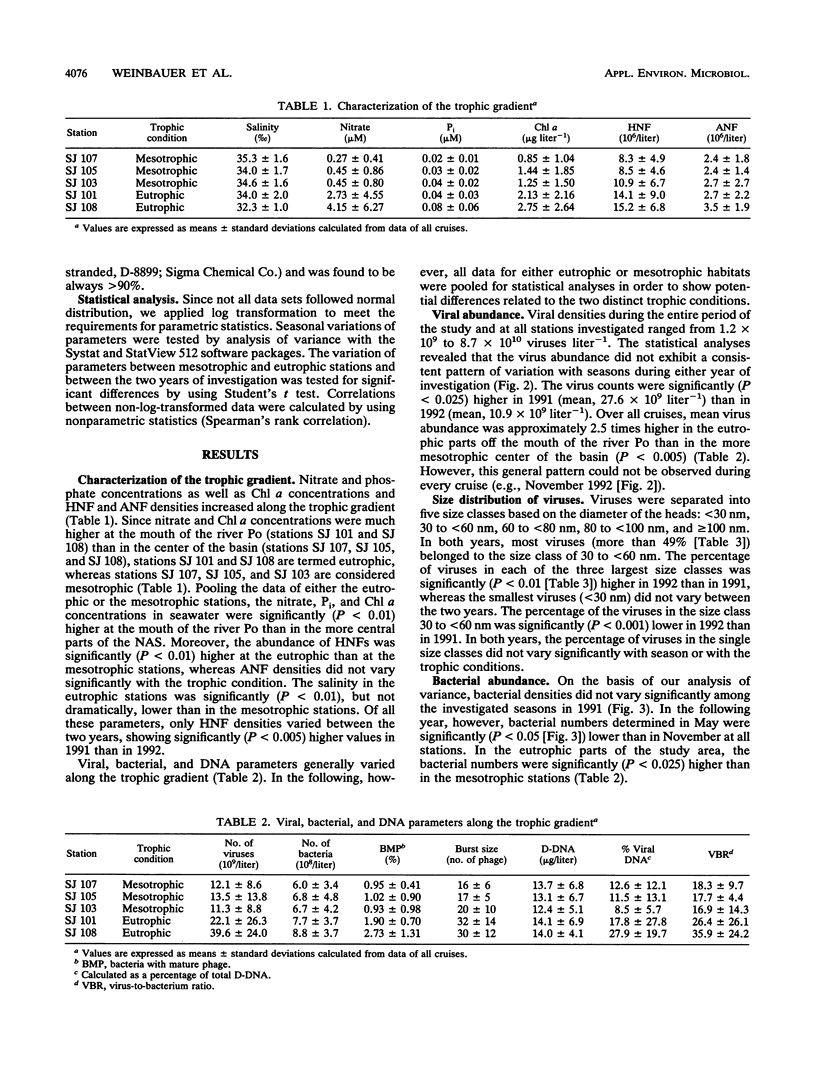
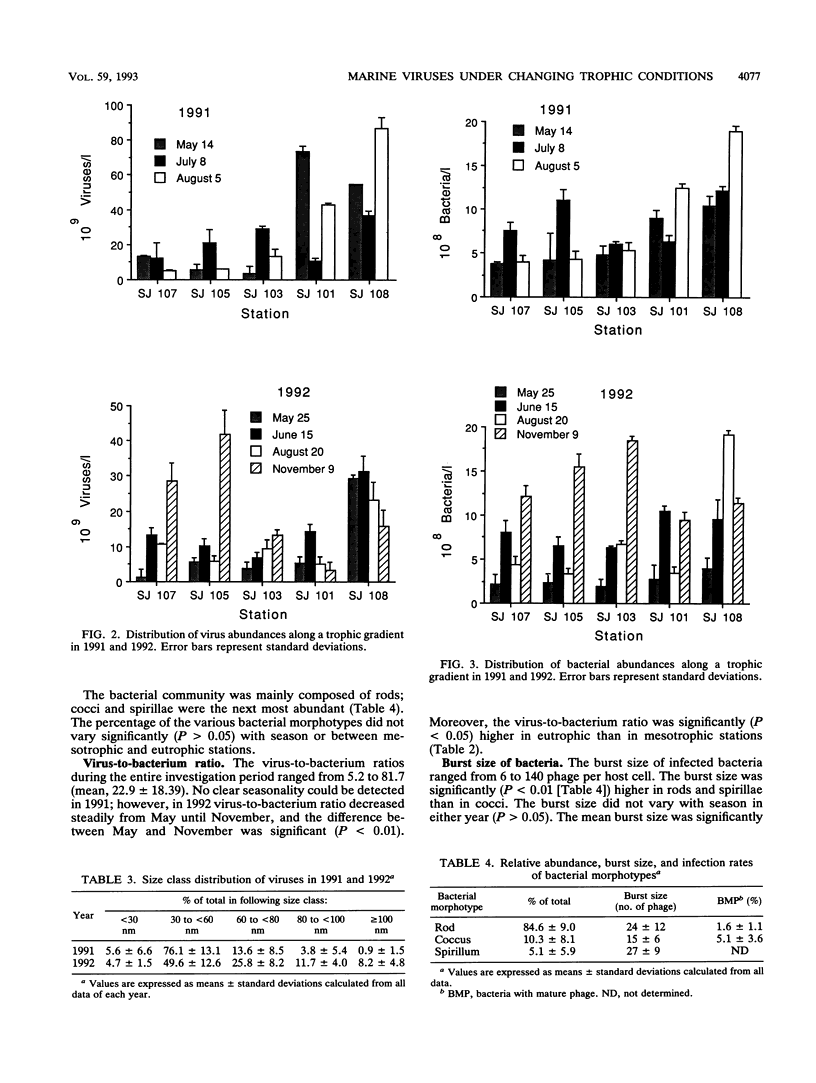
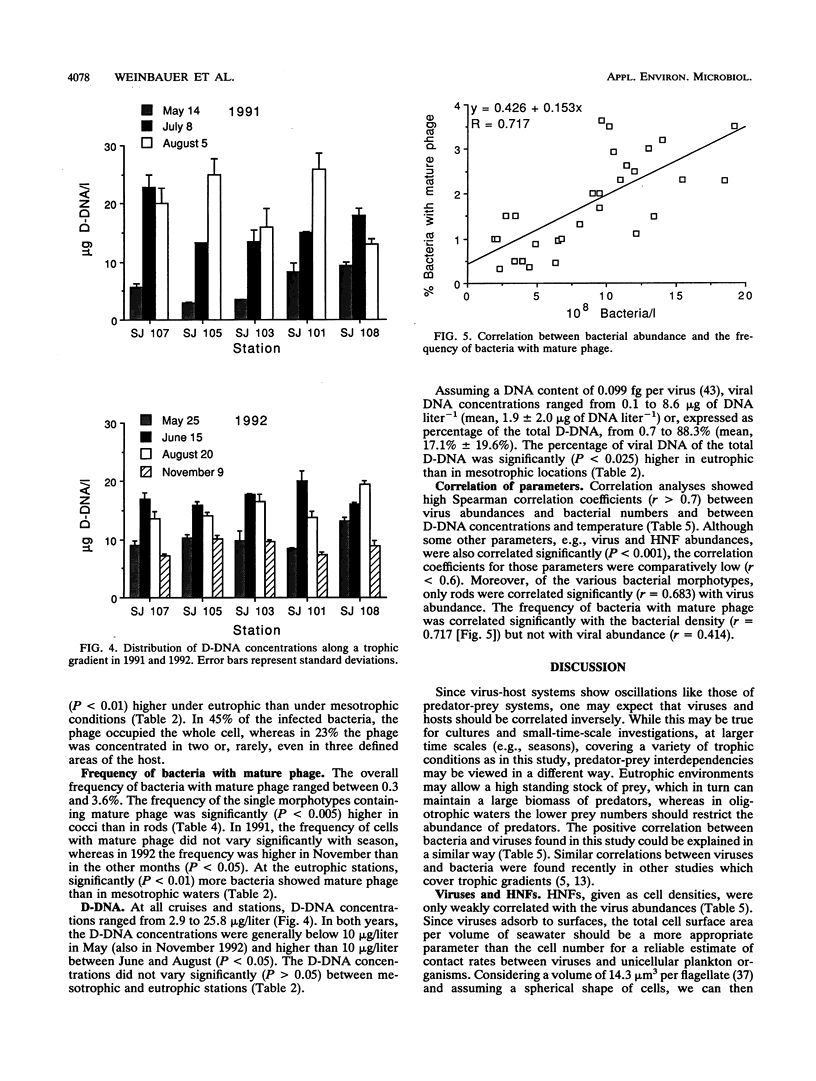
The distribution of viral and other microbial abundances as well as the concentrations of dissolved DNA (D-DNA) along a trophic gradient in the northern Adriatic Sea were determined. Virus abundances, covering a range of 1.2 × 109 to 8.7 × 1010 liter-1 were on average 2.5-fold higher in eutrophic than in mesotrophic stations. A 2.5-fold enrichment was also measured for chlorophyll a concentrations, whereas the densities of bacteria and heterotrophic nanoflagellates were only approximately 1.5-fold higher. The frequency of bacteria containing mature phage increased linearly with bacterial abundance. Assuming that mature phage is only visible during the last 14 to 27% of the latent period (L. M. Proctor, A. Okubo, and J. A. Fuhrman, Microb. Ecol. 25:161-182, 1993), we estimated that between 3.5 and 7.3% of the bacterial population was infected at mesotrophic stations versus between 7.0 and 19.5% at eutrophic stations, indicating that the bacterial mortality due to viral lysis might increase with the degree of eutrophication. The frequency of bacteria with mature phage and the burst size varied significantly with the bacterial morphotype; rod-shape cells, the most abundant morphotype, showed low infection rates but a high burst size. Concentrations of D-DNA varied significantly with season but not with trophic conditions. The estimated percentage of viral DNA on total D-DNA concentrations averaged 17.1% (range, 0.7 to 88.3%). Some kind of interaction between heterotrophic nanoflagellates and viruses is proposed. We conclude (i) that the significance of viruses varies with changing trophic conditions and (ii) that viral activity may play a significant role in food web structure under changing trophic conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bratbak G., Heldal M., Norland S., Thingstad T. F. Viruses as partners in spring bloom microbial trophodynamics. Appl Environ Microbiol. 1990 May;56(5):1400–1405. doi: 10.1128/aem.56.5.1400-1405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børsheim K. Y., Bratbak G., Heldal M. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol. 1990 Feb;56(2):352–356. doi: 10.1128/aem.56.2.352-356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. A. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol. 1983 Aug;46(2):491–498. doi: 10.1128/aem.46.2.491-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. M., Sherr E. B., Sherr B. F. Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol. 1990 Mar;56(3):583–589. doi: 10.1128/aem.56.3.583-589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S., Terauchi K., Koike I. Abundance of viruses in marine waters: assessment by epifluorescence and transmission electron microscopy. Appl Environ Microbiol. 1991 Sep;57(9):2731–2734. doi: 10.1128/aem.57.9.2731-2734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama A., Oda M., Higashihara T. Abundance of Virus-Sized Non-DNase-Digestible DNA (Coated DNA) in Eutrophic Seawater. Appl Environ Microbiol. 1993 Mar;59(3):712–717. doi: 10.1128/aem.59.3.712-717.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J., Buthala D. A. Centrifugal sedimentation of virus particles for electron microscopic counting. J Virol. 1970 May;5(5):598–603. doi: 10.1128/jvi.5.5.598-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Jeffrey W. H., DeFlaun M. F. Dynamics of extracellular DNA in the marine environment. Appl Environ Microbiol. 1987 Jan;53(1):170–179. doi: 10.1128/aem.53.1.170-179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Jiang S. C., Rose J. B. Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl Environ Microbiol. 1991 Aug;57(8):2197–2204. doi: 10.1128/aem.57.8.2197-2204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Rose J. B., Jiang S. C., Kellogg C. A., Dickson L. Distribution of viral abundance in the reef environment of Key Largo, Florida. Appl Environ Microbiol. 1993 Mar;59(3):718–724. doi: 10.1128/aem.59.3.718-724.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reanney D. C., Ackermann H. W. Comparative biology and evolution of bacteriophages. Adv Virus Res. 1982;27:205–280. doi: 10.1016/s0065-3527(08)60436-4. [DOI] [PubMed] [Google Scholar]
- Suttle C. A., Chen F. Mechanisms and rates of decay of marine viruses in seawater. Appl Environ Microbiol. 1992 Nov;58(11):3721–3729. doi: 10.1128/aem.58.11.3721-3729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk V., Rehnstam A. S., Lundberg E., Hagström A. Release of bacterial DNA by marine nanoflagellates, an intermediate step in phosphorus regeneration. Appl Environ Microbiol. 1992 Nov;58(11):3744–3750. doi: 10.1128/aem.58.11.3744-3750.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985 Jan;49(1):19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wommack K. E., Hill R. T., Kessel M., Russek-Cohen E., Colwell R. R. Distribution of viruses in the Chesapeake Bay. Appl Environ Microbiol. 1992 Sep;58(9):2965–2970. doi: 10.1128/aem.58.9.2965-2970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


