Abstract
Endothelial P-selectin expression contributes to the first wave of neutrophil (polymorphonuclear leukocyte; PMN) influx in several inflammatory conditions. Although remote tissue ischemia, such as a crush injury to the hindlimb, may result in P-selectin-mediated pulmonary leukosequestration, it is not known whether the lungs exhibit a similar response after hypothermic preservation or when subjected to a direct ischemic insult. To determine if P-selectin may mediate early primary graft failure, left lungs harvested from male Lewis rats were preserved for 6 hr at 4°C and transplanted orthotopically into isogeneic recipients. Recipients immunodepleted of PMNs before transplantation demonstrated improved graft function; pulmonary vascular resistance was reduced ≈6-fold, arterial oxygenation was increased ≈3-fold, and recipient survival was increased ≈4-fold (P < 0.05, 0.05, and 0.005, respectively). Administration of a blocking anti-P-selectin IgG 10 min before reperfusion diminished graft PMN infiltration and resulted in improved graft function and recipient survival compared with controls. To establish the role of P-selectin in normothermic pulmonary ischemia, mice were subjected to temporary left pulmonary artery ligation. After functional removal of the nonischemic right lung, mice deletionally mutant for the P-selectin gene (P-selectin −/−) exhibited reduced PMN infiltration (≈2-fold), improved arterial oxygenation (≈2-fold), and improved survival (≈3-fold) compared with P-selectin +/+ control mice (P < 0.05, 0.01, and 0.05, respectively). These studies isolate and identify the central role of a single gene product (P-selectin) in early PMN recruitment and tissue injury after frank pulmonary ischemia and in the setting of lung transplantation after hypothermic preservation.
Local P-selectin expression participates in neutrophil (polymorphonuclear leukocyte; PMN) recruitment and tissue damage in settings of acute inflammatory lung injury, but it is not known whether frank pulmonary ischemia triggers P-selectin-dependent tissue injury. Although there are multiple overlapping mechanisms by which PMNs adhere to activated endothelium (1–5), the rapidity with which PMNs accumulate in a poorly preserved pulmonary graft suggests that P-selectin-mediated PMN–endothelial interactions might be involved. P-selectin, a membrane-spanning glycoprotein that mediates the initial decelerating adhesion between PMNs and endothelial cells, may be rapidly translocated to the endothelial cell plasmalemma from a preformed storage pool within Weibel–Palade bodies (6–9). Because of the rich vascularity of the lungs, this translocation is likely to be an especially important problem during lung transplantation. In addition to P-selectin expression, which may occur during an intravascular decline in oxygen tension during organ preservation (10), aerated and reperfused lungs generate a veritable firestorm of reactive oxygen intermediates, which may themselves trigger Weibel–Palade body exocytosis (9).
In the heart (10–12) and brain (13), reperfusion injury can be attenuated by strategies that reduce selectin- or integrin-mediated PMN adhesion. However, factors governing PMN–endothelial interactions in the lungs may not be the same as those in the systemic vascular bed (14). In the case of integrins, for instance, CD18-dependent PMN emigration within the lungs was shown to be stimulus-specific, in contrast to the observation that antibody to CD18 blocked PMN emigration in the systemic vascular bed regardless of the inciting stimulus (15). Although in the setting of cobra venom factor administration (16, 17), the lungs exhibit P-selectin- and PMN-dependent tissue damage, the P-selectin-dependence of tissue injury in the lungs subjected to a direct ischemic insult is not known. In a rat model of ischemia reperfusion injury involving the crural muscle mass, local injury to the muscle was found to be P-selectin-independent (18). However, in this same model, remote injury to the lungs was noted to be P-selectin-dependent, but only under certain conditions (18). In another model of remote (intestinal) ischemia reperfusion, pulmonary microvascular injury appears to be P-selectin-dependent (19). These data underscore the fact that the selectin requirements for PMN adhesion may vary depending on the vascular bed and conditions under study.
The current studies were undertaken to gain insights into pathophysiologic mechanisms that may be operative within the vasculature of lungs subjected to a direct ischemic insult, as may occur in a variety of clinical settings, such as lung preservation for transplantation, cardiopulmonary bypass for cardiac operations, routine lung surgery, or diseases such as pulmonary embolism. In certain instances, such as primary lung graft failure, the reasons for graft failure are often unexplained, but the consequences are dire (20). To test whether early pulmonary graft dysfunction might be, at least in part, P-selectin-dependent, experiments were performed using a monoclonal antibody directed against a functional epitope of P-selectin in a rat lung preservation/transplantation model, as well as in a murine pulmonary ischemia/reperfusion model using mice deletionally mutant for the P-selectin gene.
METHODS
Rat Orthotopic Left Lung Transplant Model.
Inbred male Lewis rats (250–300 gm) were used for all experiments according to a protocol approved by the Institutional Animal Care and Use Committee at Columbia University, in accordance with guidelines of the American Association for the Accreditation of Laboratory Animal Care. Donor rats were given 500 units of heparin intravenously, and the pulmonary artery (PA) was flushed with a 30-ml volume of 4°C preservation solution at a constant pressure of 20 mmHg (1 mmHg = 133 Pa). The left lung was then harvested, a cuff was placed on each vascular stump, a cylinder was inserted into the bronchus, and the lung was submerged for 6 hr in 4°C preservation solution. For all experiments, the preservation solution consisted of modified Euro-Collins solution (Baxter Healthcare, Mundelein, IL; 10 meq/liter Na+/115 meq/liter K+/15 meq/liter Cl−/85 meq/liter HPO42−/15 meq/liter H2PO4−/10 meq/liter HCO3−), modified by adding 10 ml of 10% magnesium sulfate and 50 ml of 50% glucose solution per 1000 ml. Gender/strain/size-matched rats were anesthetized, intubated, and ventilated with 100% O2 using a rodent ventilator (Harvard Apparatus). Orthotopic left lung transplantation (21) was performed through a left thoracotomy using a rapid cuff technique for all anastomoses, with warm ischemic times maintained below 5 min. The hilar cross-clamp was then released, reestablishing blood flow and ventilation to the transplanted lung. The right lung was then functionally removed from the recipient immediately after left lung implantation. This was accomplished by passing a loose ligature around the right PA, introducing Millar catheters (2F; Millar Instruments, Houston) into the main PA and the left atrium, and placing a Doppler flow probe (Transonics, Ithaca, NY) around the main PA. The ligature was then tightened after baseline measurements were recorded (see below). The reason we chose to functionally eliminate the unaffected (right) lung was as follows. In preliminary studies, when the right lung was left functionally intact, we were unable to discriminate differences in PA flow, arterial oxygenation, or recipient survival related to the severity of the ischemic insult to the left lung (caused by increasing the duration of hypothermic preservation). Only in those instances in which the right pulmonary artery was ligated were we able to discriminate degrees of tissue injury to the left lung, and therefore, for the experiments presented in this paper, we employ the lung transplant model in which the right lung is functionally removed after left lung transplantation.
For certain experiments, recipient rats were immunodepleted of PMNs by a single intravenous injection of polyclonal rabbit anti-rat PMN antibody (α-PMN, ≈1.0 mg/kg, Accurate Scientific, Westbury, NY; refs. 10 and 22) 24 hr before transplantation. PMN depletion in these animals was confirmed by counting remaining PMNs, identified on Wright–Giemsa-stained smears of peripheral blood. In other experiments, blocking anti-P-selectin monoclonal IgG (α-PS, ≈1.0 mg/kg of monoclonal antibody PB1.3 generously provided by Cytel, San Diego; refs. 11 and 18), nonblocking anti-P-selectin monoclonal IgG [NBα-PS, ≈1.0 mg/kg of monoclonal antibody AC1.2 (10, 23, 24), Becton Dickinson], or nonimmune mouse IgG (control, ≈1.0 mg/kg, Sigma) was used. These antibodies were given intravenously to the recipient 10 min before reperfusion.
Online hemodynamic monitoring was accomplished using MacLab and a Macintosh IIci computer. Measured hemodynamic parameters included left atrium and PA pressures (millimeters of mercury), and PA flow (milliliters per minute). Arterial oxygen tension (PO2, millimeters of mercury) was measured during inspiration of 100% O2 using a model ABL-2 gas analyzer (Radiometer, Copenhagen) from a 0.3-ml sample of left atrial blood. Pulmonary vascular resistance was calculated as (mean PA pressure − left atrium pressure)/mean PA flow and expressed as millimeters of mercury per milliliter per minute. After baseline measurements, the native right PA was ligated, and serial hemodynamic measurements were taken every 5 min until the time of euthanasia at 30 min (or until recipient death). In addition to hemodynamic measurements, blood for arterial blood gas analysis was sampled at baseline and at the final time point. Neither the surgical procedure itself nor this amount of blood sampling was associated with significant blood loss, either by visual inspection or by serial measurements of hematocrits in eight representative animals (hematocrit 46.1 ± 0.5 to 46.5 ± 0.9 at the start and conclusion of surgery, respectively, P = not significant).
Murine Pulmonary Ischemia and Reperfusion Model.
To study the role of a single gene product in pulmonary ischemia using transgenic mice, we developed a new murine model of pulmonary ischemia and reperfusion. P-selectin null mice (P-selectin −/−) and their wild-type controls (P-selectin +/+; provided by D. Wagner; ref. 25) were used in these experiments according to a protocol approved by the Institutional Animal Care and Use Committee at Columbia University, in accordance with guidelines of the American Association for the Accreditation of Laboratory Animal Care. After appropriate anesthesia, mice were placed on a Harvard ventilator (tidal volume = 0.75 ml, respiratory rate = 180/min) and ventilated with 100% O2, and underwent bilateral thoracotomy. The left pulmonary artery was cross-clamped for a period of 30 or 60 min, the cross-clamp was released, and then the contralateral (right) PA was permanently ligated, so that the animal’s survival and gas exchange depended solely upon the reperfused lung. Lung function was ascertained by arterial blood gas analysis (sampled from the left ventricle) 30 min after reperfusion or at the time of recipient death, and survival was recorded at the 30-min time point after ligation of the right PA.
Myeloperoxidase Assay.
Thirty minutes after ligation of the native right PA, or at the time of recipient death, transplanted (rat) or ischemic/reperfused (mouse) lungs were removed, rinsed briskly in physiologic saline, and snap-frozen in liquid nitrogen until the time of the myeloperoxidase assay. Tissue was homogenized in phosphate buffer (50 mM, pH 5.5) containing hexadecyltrimethyl ammonium bromide (HTAB, 0.5%, Sigma). The assay was performed, as described (26), by thawing the sample, centrifuging at 40,000 × g for 15 min, and decanting the supernatant, which was assayed for myeloperoxidase activity using a standard chromogenic spectrophotometric technique in which test sample (0.03 ml) was added to phosphate buffer (0.97 ml) containing O-dianisidine dihydrochloride (Sigma) and hydrogen peroxide (0.0005%). Change in absorbance at 460 nm was measured over 1 min (increase in OD was linear over this time interval). Rat lungs were sufficiently large to add 5 ml/g HTAB buffer to tissue before homogenization, so that myeloperoxidase activity was expressed as ΔA460/min. For mouse lungs, 2 ml of HTAB buffer was added to each sample. In this latter case, protein concentration after homogenization was measured, and myeloperoxidase activity was expressed as ΔA460/min/mg of protein.
Statistics.
The Mann–Whitney U test was used to compare different conditions. Animal survival data were analyzed by contingency analysis using the χ2 statistic. Values are expressed as the mean ± SEM, with differences considered statistically significant if P < 0.05.
RESULTS
Rat Lung Transplant Model.
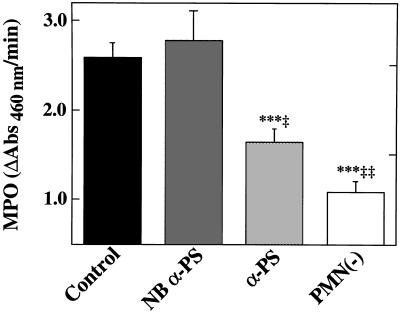
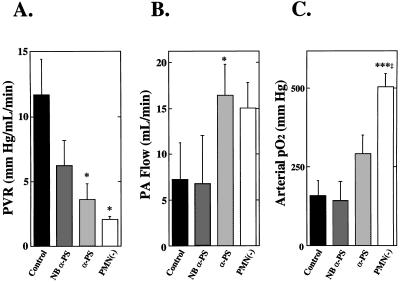
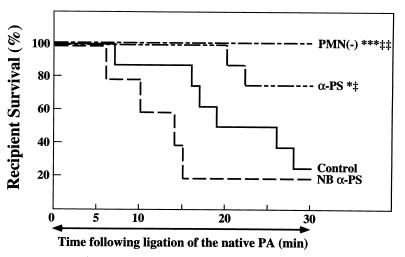
To determine whether subjecting a richly vascularized organ such as the lungs to hypothermic preservation and transplantation may result in P-selectin-dependent PMN adhesion and pulmonary dysfunction, experiments were performed in an orthotopic rat left lung transplant model in which the right lung was functionally removed after left lung implantation (21). This model was adopted based upon published procedures (21, 27–30), as well as preliminary data demonstrating that, if the right lung were left intact, then the severity of the ischemic insult to the left lung could not be gauged by pulmonary functional or survival parameters, due to compensation from the relatively larger/unaffected right lung. To demonstrate that PMNs were responsible, at least in part, for early graft failure in lungs subjected to prolonged (6-hr) hypothermic preservation, experiments were performed using PMN-depleted recipient rats. The polyclonal rabbit anti-rat PMN used in these experiments eliminated virtually all circulating PMNs in the recipients, with little effect on other cell types (10, 22). When lungs were transplanted into the PMN-free reperfusion milieu, graft myeloperoxidase activity was significantly reduced compared with grafts transplanted into control recipients that had received nonimmune IgG 10 min before reperfusion (Fig. 1). In parallel with reduced graft PMN infiltration, recipient PMN depletion significantly reduced pulmonary vascular resistance (Fig. 2A), increased PA flow (Fig. 2B), improved arterial oxygenation (Fig. 2C), and improved recipient survival (Fig. 3) after lung transplantation. To determine whether the benefit of recipient neutrophil depletion persisted beyond the 30-min observation period, additional experiments were performed in neutrophil-depleted recipients which were not euthanized at 30 min. These experiments (n = 4) demonstrated a mean survival time of 6.0 ± 2.4 hr after transplantation and ligation of the right PA (these animals required additional doses of anesthetic agents during the extended observation period).
Figure 1.
Effects of recipient neutropenia or P-selectin blockade on graft PMN accumulation. All lung transplants were performed after 6 hr of hypothermic preservation in Euro-Collins solution. Neutropenia was established by immunodepleting recipient PMNs using an anti-rat PMN antibody given intravenously 24 hr before transplantation [PMN(−), n = 6]. P-selectin blockade was accomplished using a blocking anti-P-selectin IgG given intravenously to recipients 10 min before reperfusion (α-PS, n = 8). Comparison is made with controls treated with nonimmune IgG (Control, n = 8) or recipients treated with isotype-matched NBα-PS (n = 5). Myeloperoxidase activity (MPO; ΔAbs 460 nm/min) was used to quantify graft PMN deposition. Data are presented as the mean ± SEM. ∗∗∗, P < 0.005 vs. Control; ‡, P < 0.05 and ‡‡, P < 0.01 vs. NBα-PS.
Figure 2.
Effects of recipient neutropenia or P-selectin blockade on hemodynamic and functional indices of lung preservation. Conditions are identical to those described in the legend to Fig. 1. After ligation of the native right pulmonary artery, measurements were recorded at the final time at which the recipient was alive or at 30 min if the animal survived to that time. (A) Pulmonary vascular resistance. (B) Pulmonary arterial flow. (C) Arterial oxygenation. Data are presented as the mean ± SEM. Controls, n = 8; NBα-PS, n = 5; α-P-selectin, n = 8; PMN(−), n = 6. ∗, P < 0.05 and ∗∗∗, P < 0.005 vs. Control; ‡, P < 0.05 vs. NBα-PS.
Figure 3.
Effects of recipient neutropenia or P-selectin blockade on recipient survival. Lung transplants were performed in nonimmune IgG-treated recipients (Control, n = 8), recipients treated with NBα-PS (n = 5) or blocking anti-P-selectin antibody (α-P-selectin, n = 8), or recipients immunodepleted of PMNs [PMN(−), n = 6]. Experiments were performed as described in the legend to Fig. 1. ∗, P < 0.05 and ∗∗∗, P < 0.005 vs. Control; ‡, P < 0.05 and ‡‡, P < 0.01 vs. NBα-PS.
To establish the P-selectin dependence of early posttransplant PMN accumulation and tissue damage, separate experiments were performed in which recipient rats were infused with a functionally blocking anti-P-selectin IgG 10 min before reperfusion. Grafts transplanted into recipients that had received this blocking antibody showed reduced graft myeloperoxidase activity (Fig. 1) and improved graft function (Fig. 2) compared with either control rats which received nonimmune IgG or rats that had received a nonblocking anti-P-selectin antibody before transplantation. In parallel with these functional improvements, rats which had received the blocking anti-P-selectin IgG demonstrated improved survival (Fig. 3).
Murine Model of Pulmonary Ischemia and Reperfusion.
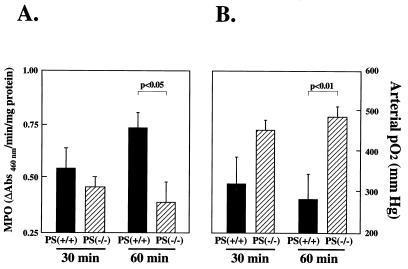
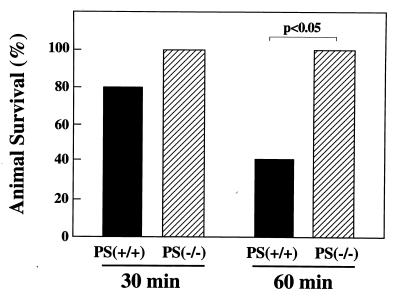
To define the role of P-selectin after normothermic pulmonary ischemia and reperfusion, experiments were performed using a murine model. Homozygous null P-selectin mice (P-selectin −/−; ref. 25) which were subjected to 30 min of pulmonary ischemia followed by reperfusion showed a trend toward reduced PMN infiltration (Fig. 4A), improved arterial oxygenation (Fig. 4B), and improved survival (Fig. 5). When the ischemic duration was extended to 60 min, the P-selectin −/− mice showed significant protection from pulmonary ischemia and reperfusion injury, demonstrated by significant reductions in PMN infiltration, improved arterial oxygenation, and improved survival compared with wild-type (P-selectin +/+) mice subjected to identical procedures. (Figs. 4 and 5). When additional experiments (n = 6) were performed to determine whether P-selectin −/− mice subjected to the more stringent (60 min) ischemic protocol followed by right PA ligation could survive beyond 30 min, mean survival time was 83.3 ± 16 min. Again, as for the longer observation experiments in rats, mice subjected to 60 min ischemia and extended observation required repeated doses of anesthetic agents during the course of experiments.
Figure 4.
Effect of P-selectin gene expression on lung PMN sequestration (A) and function (B) after frank pulmonary ischemia and reperfusion. A murine pulmonary ischemia and reperfusion model was used; in this model, the left pulmonary artery was cross-clamped for a period of 30 or 60 min, after which the cross-clamp was released and the contralateral (right) pulmonary artery was permanently ligated. PMN infiltration into the postischemic lung was measured as myeloperoxidase activity as described in Methods. Arterial oxygenation (pO2) was measured from a sample of left atrial blood. Tissue and blood samples were obtained 30 min after ligation of the right (nonischemic) pulmonary artery, or just before death if the animal failed to survive for 30 min [n = 5 each for P-selectin null mice (P-selectin −/−) and wild-type controls (P-selectin +/+) at 30 min ischemia, and n = 10 each for P-selectin −/− and P-selectin +/+ mice at 60 min ischemia). Data are presented as the mean ± SEM.
Figure 5.
Effect of P-selectin gene expression on mouse survival after frank pulmonary ischemia and reperfusion. Ischemic durations are shown. Animal survival was recorded at the 30-min time point after ligation of the right (nonischemic) pulmonary artery [n = 5 each for P-selectin null mice (P-selectin −/−) and wild-type controls (P-selectin +/+) at 30 min ischemia, and n = 10 each for P-selectin −/− and P-selectin +/+ mice at 60 min ischemia). Data are presented as the mean ± SEM.
DISCUSSION
Neutrophil recruitment into postischemic loci entails a series of overlapping adhesion mechanisms that are somewhat redundant (1–5). It is often not clear which molecules are the critical mediators of PMN adhesion under given conditions. For both integrins (15) and selectins (18, 19), there are instances in which the stimuli that induce PMN adhesion vary depending upon the vascular bed under study. Although interleukin-8, P-selectin, and intercellular adhesion molecule-1 function as important inflammatory mediators in a variety of settings (10, 13, 31–34), the pathophysiological role of P-selectin in the postischemic lungs has received little attention. P-selectin, a membrane-spanning glycoprotein expressed by activated endothelial cells and platelets, binds to sialylated carbohydrate ligands on PMNs to decelerate them in their rapid transit through the vasculature. In a murine model of peritoneal inflammation, failure to express P-selectin delays PMN accumulation and subsequent peritonitis but does not otherwise modify the clinical course (25). In the heart, P-selectin expression appears to be an important mediator of PMN adhesion and tissue injury after ischemia (10, 11). Although P-selectin mediates PMN capture in the lungs after intravenous infusion of cobra venom factor (16, 17) or after ischemia in remote regions such as the hindlimb (18) or gut (19), the role of P-selectin expression after hypothermic pulmonary preservation or frank pulmonary ischemia has not been previously reported. The current studies are the first, to our knowledge, to demonstrate not only that P-selectin participates in PMN capture after normothermic or hypothermic pulmonary ischemia, but also that this participation is pathophysiologically significant.
There are a number of relevant stimuli during pulmonary ischemia or reperfusion that may trigger P-selectin expression (9, 10, 16–19). P-selectin, stored in subplasmalemmal Weibel–Palade bodies (6), may be rapidly expressed at the endothelial surface after a period of oxygen deprivation (10) or in an oxidant-rich vascular milieu (9). These observations may be particularly relevant to lung transplantation, during which there is a transition from profound ischemia during circulatory arrest and preservation to one in which there are abundant reactive oxygen species in the aerated and reperfused lungs after transplantation. Although oxygen-starved endothelial cells undergo striking phenotypic modulation to become prothrombotic and proinflammatory, many of these processes require de novo protein synthesis (31, 35, 36). P-selectin, which is largely preformed, is therefore a likely molecular candidate to participate in early PMN capture after hypothermic lung preservation. Although the current studies do not rule out important roles of other cell adhesion receptors, they do identify a critical pathophysiological role for P-selectin in early posttransplant lung graft failure. Furthermore, the experiments in which P-selectin null mice demonstrate significant protection against early tissue injury after normothermic pulmonary ischemia help to clearly identify the role of a single gene product, P-selectin, in the pathophysiology of lung ischemia and reperfusion.
One technical limitation of the current study is related to the extreme stringency of the surgical conditions. In the left lung transplant model, failing to ligate the right PA prohibits discrimination of the functional severity of injury to the transplanted (left) lung. Therefore, because in the model used for the present studies the right PA is ligated, forcing all ventilation and perfusion to the transplanted lung, this model is perhaps more relevant to double-lung than to single-lung transplantation in humans. The 30-min posttransplant observation period was selected so as to study early mediators of primary graft failure and to facilitate comparison with previously published studies (21, 27–30). Although longer survival (beyond the predefined 30-min period) was possible in both PMN-depleted transplant recipients and P-selectin-deficient mice, surgical considerations such as the need for repeated doses of anesthetic agents, self-extubation of animals, etc., make it difficult to precisely determine the outer limit of survival under these extremely stringent conditions. Nevertheless, the fact that human lung grafts can fail within minutes to hours of reperfusion (20) suggests that the time frames achieved in the current experiments can provide insights into clinically relevant potential mechanisms of primary lung graft failure.
This study contributes to the emerging data regarding the detrimental role of PMNs during the vulnerable early reperfusion period after lung transplantation (28, 30, 37, 38). There are likely to be several explanations for PMN-mediated pulmonary tissue damage. PMNs, as sentinels of the immune system, are laden with tissue-destructive enzymes and may be activated to release both toxic oxidants and acids (39). When activated, PMNs increase in size and stiffness, which facilitates their trapping in alveolar capillaries and precapillary vessels (14, 40, 41). This may contribute to the no-reflow phenomenon, wherein a tissue fails to reperfuse despite restoration of adequate perfusion pressures (42–44). Current optimal clinical pulmonary preservation strategies, which consist of hypothermic lung storage in an electrolyte solution with no form of reperfusion therapy (45), miss the chance to mitigate deleterious PMN–endothelial adhesive interactions. Not surprisingly, early and unexplained lung graft failure occurs in human lung transplantation, with dire clinical consequences (20). Data from the current study suggest that strategies that target PMN–endothelial interactions during pulmonary reperfusion can improve both early posttransplant graft function and recipient survival. Because the anti-P-selectin antibody we have used (PB1.3) has been humanized for potential clinical use (M. Laurie Phillips, personal communication), and because of the development of other novel therapeutic strategies designed to block the activity of P-selectin expressed at the endothelial surface, our studies should provide an impetus for the testing of these compounds in humans in the setting of lung ischemia or transplantation.
Acknowledgments
We gratefully acknowledge the expert assistance of Kathy Tang during the course of these studies. This work was supported in part by grants from the Cystic Fibrosis Foundation, a Grant-in-Aid from the American Heart Association, and Public Health Service Grant HL55397. M.C.O. is the recipient of a Robert E. Gross Research Scholarship and is an Irving Assistant Professor of Surgery, and D.J.P. is a Clinician-Scientist of the American Heart Association.
Footnotes
Abbreviations: HTAB, hexadecyltrimethyl ammonium bromide; NBα-PS, nonblocking anti-P-selectin antibody; PA, pulmonary artery; PMN, polymorphonuclear leukocyte.
References
- 1.Springer T A. Nature (London) 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 2.Butcher D C. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 3.Lorant D E, Topham M K, Whatley R E, McEver R P, McIntyre T M, Prescott S M, Zimmerman G A. J Clin Invest. 1993;92:559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorant D E, Patel K D, McIntyre T M, McEver R P, Prescott S M, Zimmerman G A. J Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence M B, Springer T A. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 6.Weibel E R, Palade G E. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattori R, Hamilton K K, McEver R P, Sims P J. J Biol Chem. 1989;264:9053–9060. [PubMed] [Google Scholar]
- 8.Geng J-G, Bevilacqua M P, Moore K L, McIntyre T M, Prescott S M, Kim J M, Bliss G A, Zimmerman G A, McEver R P. Nature (London) 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 9.Patel K D, Zimmerman G A, Prescott S M, McEver R P, McIntyre T M. J Cell Biol. 1991;112:749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinsky D J, Naka Y, Liao H, Oz M C, Wagner D D, Mayadas T N, Johnson R C, Hynes R O, Heath M, Lawson C A, Stern D M. J Clin Invest. 1996;97:493–500. doi: 10.1172/JCI118440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyrich A S, Ma X Y, Lefer D J, Albertine K H, Lefer A M. J Clin Invest. 1993;91:2620–2629. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefer D J, Flynn D M, Phillips M L, Ratcliffe M, Buda A J. Circulation. 1994;90:2390–2401. doi: 10.1161/01.cir.90.5.2390. [DOI] [PubMed] [Google Scholar]
- 13.Connolly E S, Jr, Winfree C J, Springer T A, Naka Y, Liao H, Yan S D, Stern D M, Solomon R A, Gutierrez-Ramos J-C, Pinsky D J. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gee M H, Albertine K H. Annu Rev Physiol. 1993;55:227–248. doi: 10.1146/annurev.ph.55.030193.001303. [DOI] [PubMed] [Google Scholar]
- 15.Doerschuk C M, Winn R K, Coxson H O, Harlan J M. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- 16.Mulligan M S, Polley M J, Bayer R J, Nunn M F, Paulson J C, Ward P A. J Clin Invest. 1992;90:1600–1607. doi: 10.1172/JCI116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulligan M S, Paulson J C, de Frees S, Zheng Z-L, Lowe J B, Ward P A. Nature (London) 1993;364:149–151. doi: 10.1038/364149a0. [DOI] [PubMed] [Google Scholar]
- 18.Seekamp A, Till G O, Mulligan M S, Paulson J C, Anderson D C, Miyasaka M, Ward P A. Am J Pathol. 1994;144:592–598. [PMC free article] [PubMed] [Google Scholar]
- 19.Carden D L, Young J A, Granger D N. J Appl Physiol. 1993;75:2529–2534. doi: 10.1152/jappl.1993.75.6.2529. [DOI] [PubMed] [Google Scholar]
- 20.Whyte R I, Deeb G M, McCurry K R, Anderson H L, III, Bolling S F, Bartlett R H. Ann Thorac Surg. 1994;58:754–759. doi: 10.1016/0003-4975(94)90741-2. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury N C, Naka Y, Pinsky D J, Yano O J, Smith C R, Rose E A, Stern D M, Michler R E, Oz M C. Surg Forum. 1994;45:268–270. [Google Scholar]
- 22.Henderson R F, Harkema J A, Hotchkiss J A, Boehme D S. Toxicol Appl Pharmacol. 1991;109:127–136. doi: 10.1016/0041-008x(91)90196-l. [DOI] [PubMed] [Google Scholar]
- 23.Larson E, Celi A, Gilbert G E, Furie B C, Erban J K, Bonfanti R, Wagner D D, Furie B. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 24.Stone J P, Wagner D D. J Clin Invest. 1993;92:804–813. doi: 10.1172/JCI116654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayadas T N, Johnson R C, Rayburn H, Hynes R O, Wagner D D. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 26.Goldblum S E, Wu K-M, Jay M. J Appl Physiol. 1978;59:1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- 27.Naka Y, Chowdhury N C, Oz M C, Smith C R, Yana O J, Michler R E, Stern D M, Pinsky D J. J Thorac Cardiovasc Surg. 1995;109:206–211. doi: 10.1016/S0022-5223(95)70380-2. [DOI] [PubMed] [Google Scholar]
- 28.Pinsky D J, Naka Y, Chowdhury N C, Liao H, Oz M C, Michler R E, Kubaszewski E, Malinski T, Stern D M. Proc Natl Acad Sci USA. 1994;91:12086–12090. doi: 10.1073/pnas.91.25.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naka Y, Roy D K, Smerling A J, Michler R E, Smith C R, Stern D M, Oz M C, Pinsky D J. J Thorac Cardiovasc Surg. 1995;110:1434–1441. doi: 10.1016/S0022-5223(95)70066-8. [DOI] [PubMed] [Google Scholar]
- 30.Naka Y, Chowdhury N C, Liao H, Roy D K, Oz M C, Michler R E, Pinsky D J. Circ Res. 1995;76:900–906. doi: 10.1161/01.res.76.5.900. [DOI] [PubMed] [Google Scholar]
- 31.Karakurum M, Shreeniwas R, Chen J, Pinsky D, Yan S D, Anderson M, Sunouchi K, Major J, Hamilton T, Kuwabara K, Rot A, Nowygrod R, Stern D. J Clin Invest. 1994;93:1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerome S N, Dore M, Paulson J C, Smith C W, Korthuis R J. Am J Physiol. 1994;266:H1316–H1321. doi: 10.1152/ajpheart.1994.266.4.H1316. [DOI] [PubMed] [Google Scholar]
- 33.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. J Leukocyte Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 34.Okada Y, Copeland B R, Mori E, Tung M M, Thomas W S, dep Zoppo G J. Stroke (Dallas) 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- 35.Shreeniwas R, Koga S, Karakurum M, Pinsky D, Kaiser E, Brett J, Wolitzky B A, Norton C, Plocinski J, Benjamin W, Burns D K, Goldstein A, Stern D. J Clin Invest. 1992;90:2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa S, Gerlach H, Esposito C, Pasagian-Macaulay A, Brett J, Stern D. J Clin Invest. 1990;85:1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall T S, Breda M A, Baumgartner W A, Borkon A M, Brawn J, Hutchins G M, Reitz B A. Curr Surg. 1987;44:137–139. [PubMed] [Google Scholar]
- 38.Pillai R, Bando K, Schueler S, Zebley M, Reitz B A, Baumgartner W A. Ann Thorac Surg. 1990;50:211–214. doi: 10.1016/0003-4975(90)90736-p. [DOI] [PubMed] [Google Scholar]
- 39.Weiss S J. N Engl J Med. 1989;89:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 40.Hogg J C. Physiol Rev. 1987;67:1249–1295. doi: 10.1152/physrev.1987.67.4.1249. [DOI] [PubMed] [Google Scholar]
- 41.Staub N C, Schultz E L, Albertine K H. Ann NY Acad Sci. 1982;384:332–343. doi: 10.1111/j.1749-6632.1982.tb21382.x. [DOI] [PubMed] [Google Scholar]
- 42.Engler R L, Schmid-Schoenbein G W, Pavelec R S. Am J Pathol. 1983;111:98–111. [PMC free article] [PubMed] [Google Scholar]
- 43.Carden D L, Smith J K, Korthuis R J. Circ Res. 1990;66:1436–1444. doi: 10.1161/01.res.66.5.1436. [DOI] [PubMed] [Google Scholar]
- 44.Del Zoppo G J, Schmid-Schoenbein G W, Mori E, Copeland B R, Chang C-M. Stroke (Dallas) 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 45.Kirk A J B, Colquhoum I W, Dark J H. Ann Thorac Surg. 1993;56:990–1000. doi: 10.1016/0003-4975(93)90378-u. [DOI] [PubMed] [Google Scholar]