Abstract
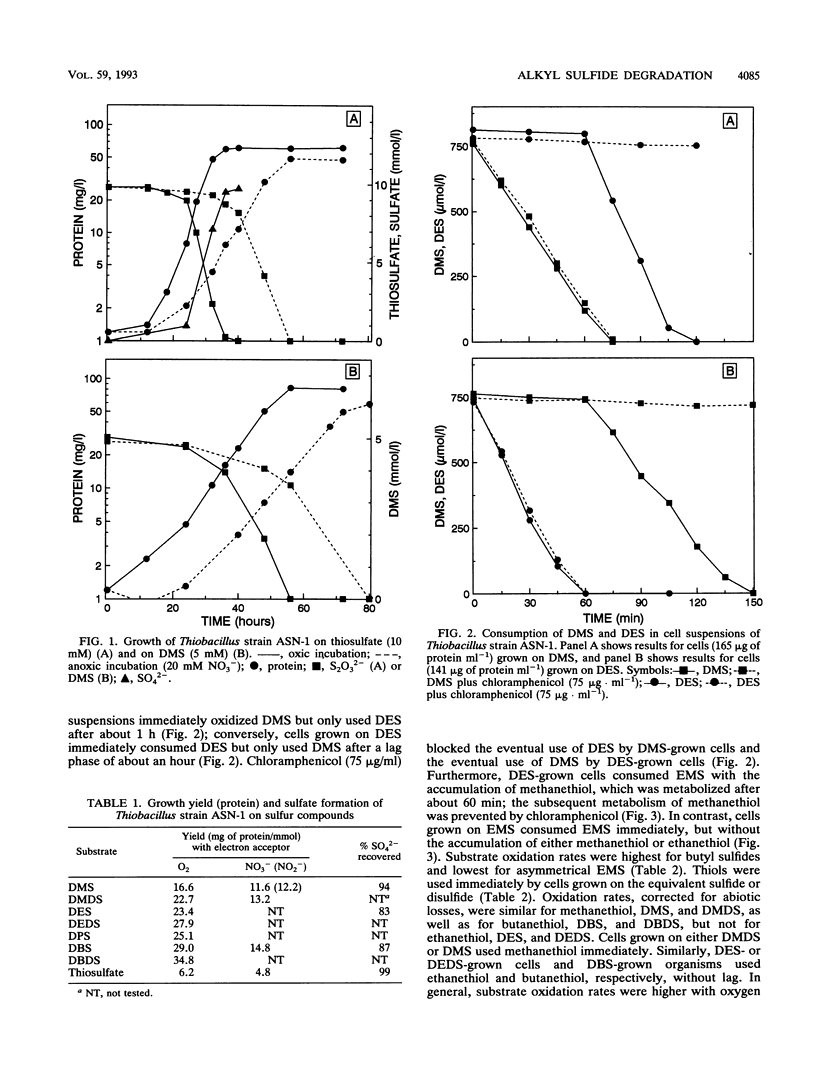
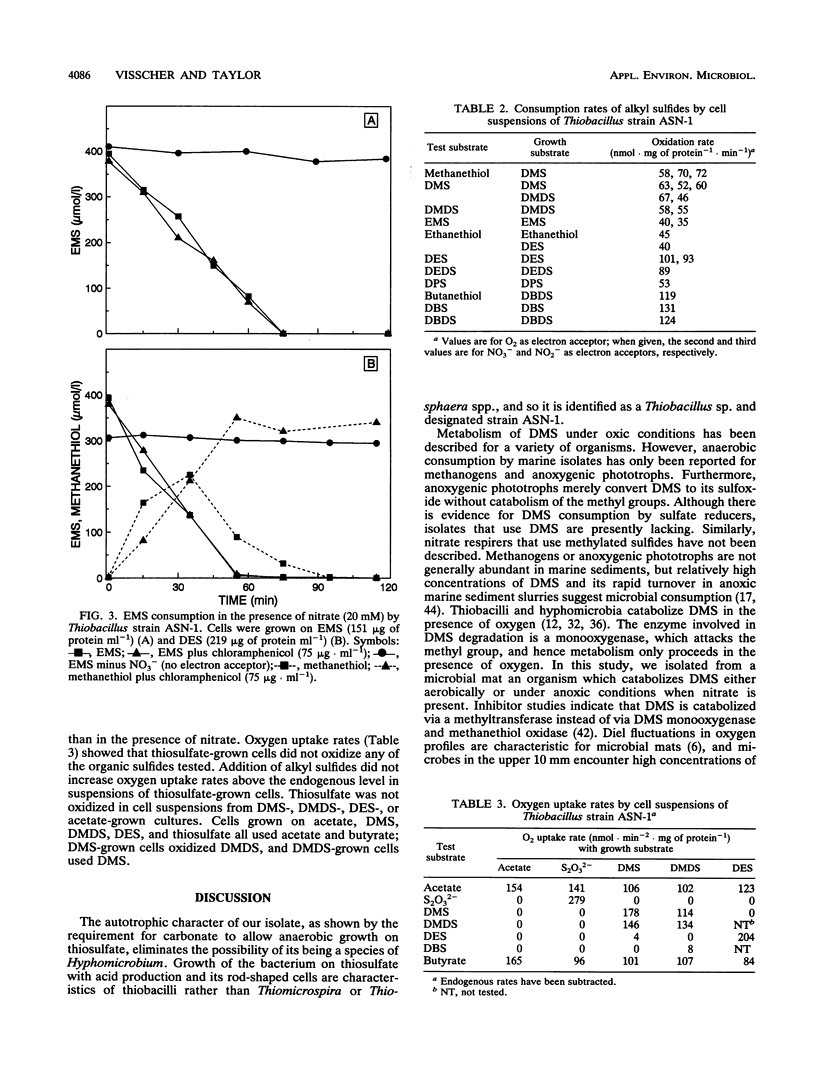
A pure culture of a bacterium was obtained from a marine microbial mat by using an anoxic medium containing dimethyl sulfide (DMS) and nitrate. The isolate grew aerobically or anaerobically as a denitrifier on alkyl sulfides, including DMS, dimethyl disulfide, diethyl sulfide (DES), ethyl methyl sulfide, dipropyl sulfide, dibutyl sulfide, and dibutyl disulfide. Cells grown on an alkyl sulfide or disulfide also oxidized the corresponding thiols, namely, methanethiol, ethanethiol, propanethiol, or butanethiol. Alkyl sulfides were metabolized by induced or derepressed cells with oxygen, nitrate, or nitrite as electron acceptor. Cells grown on DMS immediately metabolized DMS, but there was a lag before DES was consumed; with DES-grown cells, DES was immediately used but DMS was used only after a lag. Chloramphenicol prevented the eventual use of DES by DMS-grown cells and DMS use by DES-grown cells, respectively, indicating separate enzymes for the metabolism of methyl and ethyl groups. Growth was rapid on formate, acetate, propionate, and butyrate but slow on methanol. The organism also grew chemolithotrophically on thiosulfate with a decrease in pH; growth required carbonate in the medium. Growth on sulfide was also carbonate dependent but slow. The isolate was identified as a Thiobacillus sp. and designated strain ASN-1. It may have utility for removing alkyl sulfides, and also nitrate, nitrite, and sulfide, from wastewaters.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kanagawa T., Mikami E. Removal of methanethiol, dimethyl sulfide, dimethyl disulfide, and hydrogen sulfide from contaminated air by Thiobacillus thioparus TK-m. Appl Environ Microbiol. 1989 Mar;55(3):555–558. doi: 10.1128/aem.55.3.555-558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Oremland R. S., Catena A., Miller L. G., Capone D. G. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl Environ Microbiol. 1986 Nov;52(5):1037–1045. doi: 10.1128/aem.52.5.1037-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther G. W., 3rd, Church T. M., Scudlark J. R., Cosman M. Inorganic and organic sulfur cycling in salt-marsh pore waters. Science. 1986 May 9;232(4751):746–749. doi: 10.1126/science.232.4751.746. [DOI] [PubMed] [Google Scholar]
- Ni S. S., Boone D. R. Isolation and characterization of a dimethyl sulfide-degrading methanogen, Methanolobus siciliae HI350, from an oil well, characterization of M. siciliae T4/MT, and emendation of M. siciliae. Int J Syst Bacteriol. 1991 Jul;41(3):410–416. doi: 10.1099/00207713-41-3-410. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- Visscher P. T., Quist P., van Gemerden H. Methylated sulfur compounds in microbial mats: in situ concentrations and metabolism by a colorless sulfur bacterium. Appl Environ Microbiol. 1991 Jun;57(6):1758–1763. doi: 10.1128/aem.57.6.1758-1763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher P. T., Taylor B. F. A new mechanism for the aerobic catabolism of dimethyl sulfide. Appl Environ Microbiol. 1993 Nov;59(11):3784–3789. doi: 10.1128/aem.59.11.3784-3789.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher P. T., Taylor B. F. Organic thiols as organolithotrophic substrates for growth of phototrophic bacteria. Appl Environ Microbiol. 1993 Jan;59(1):93–96. doi: 10.1128/aem.59.1.93-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher P. T., van Gemerden H. Production and consumption of dimethylsulfoniopropionate in marine microbial mats. Appl Environ Microbiol. 1991 Nov;57(11):3237–3242. doi: 10.1128/aem.57.11.3237-3242.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]


