Abstract
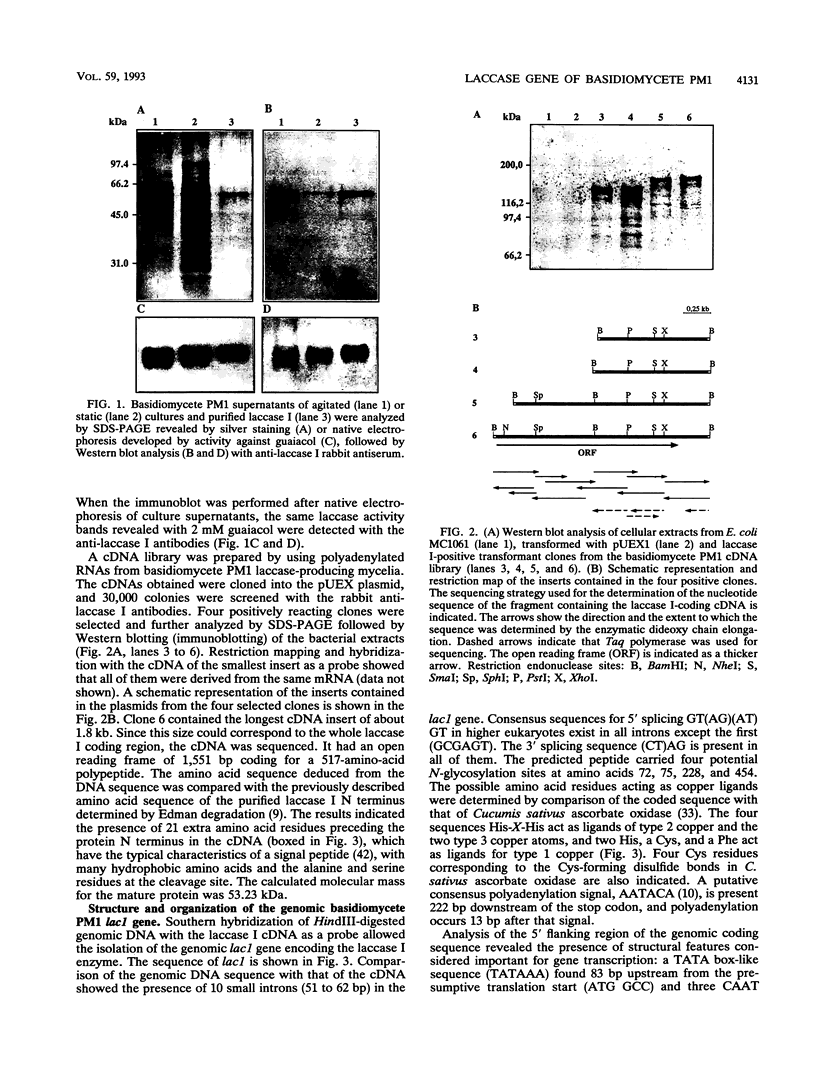
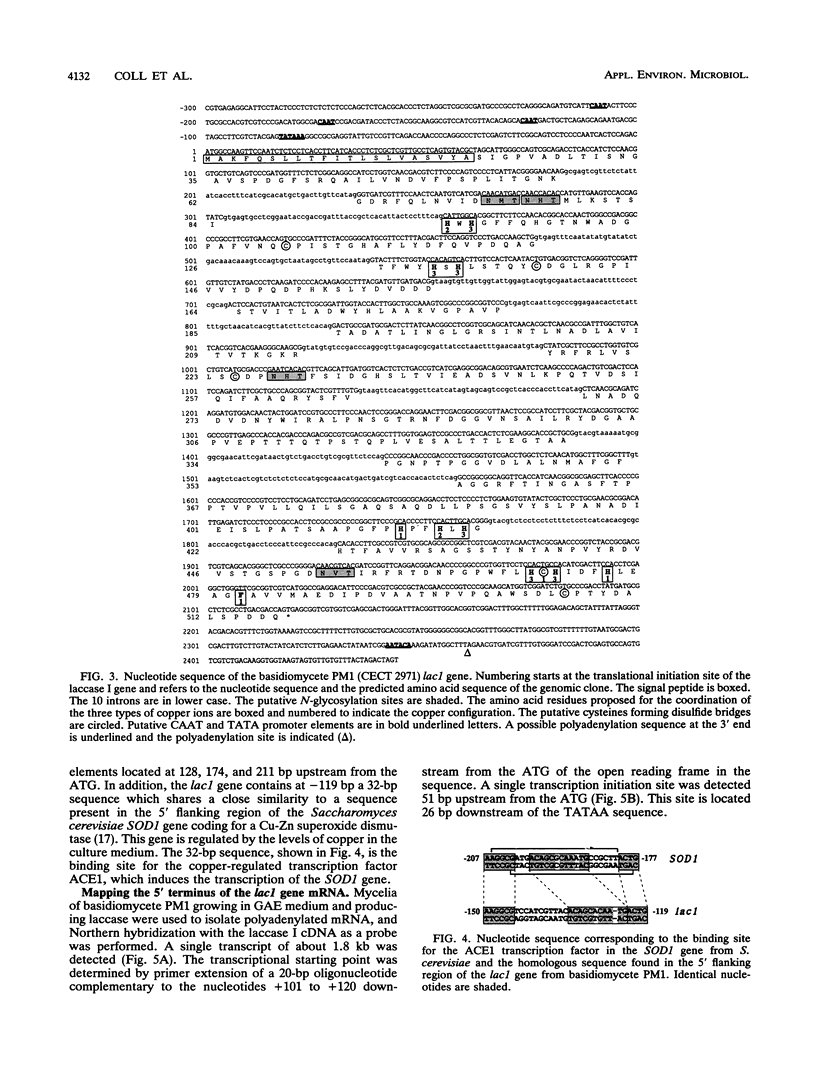
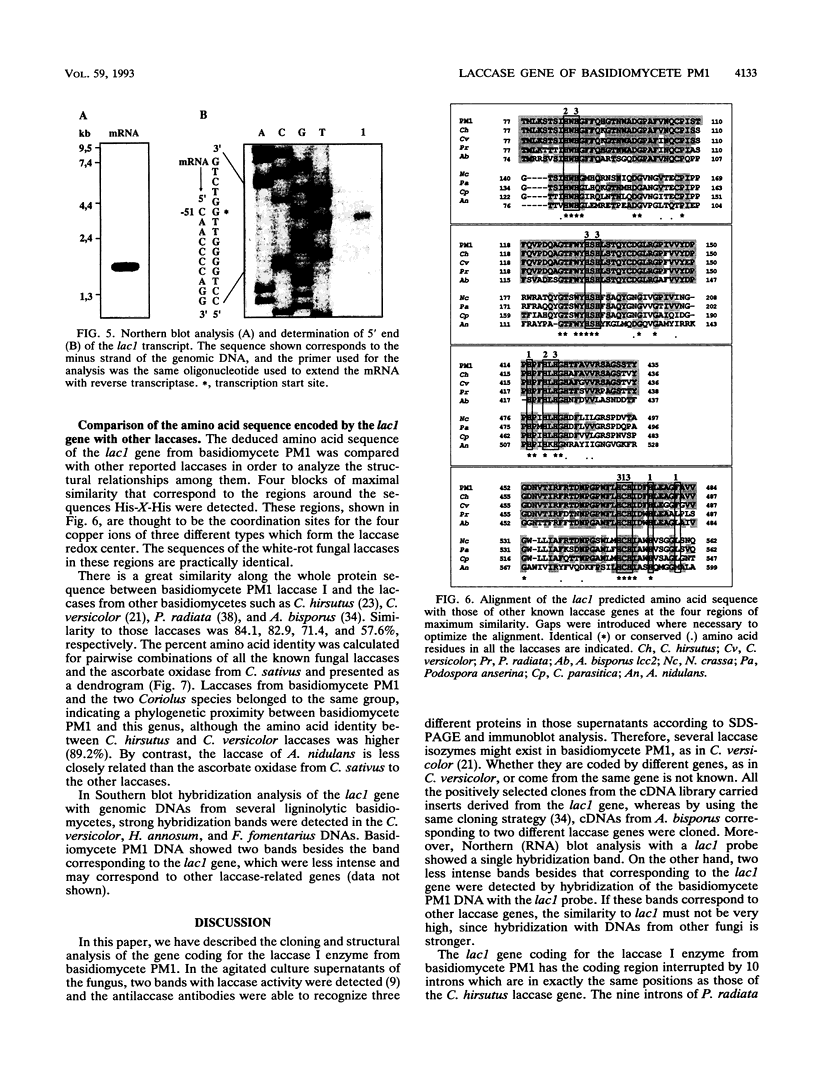
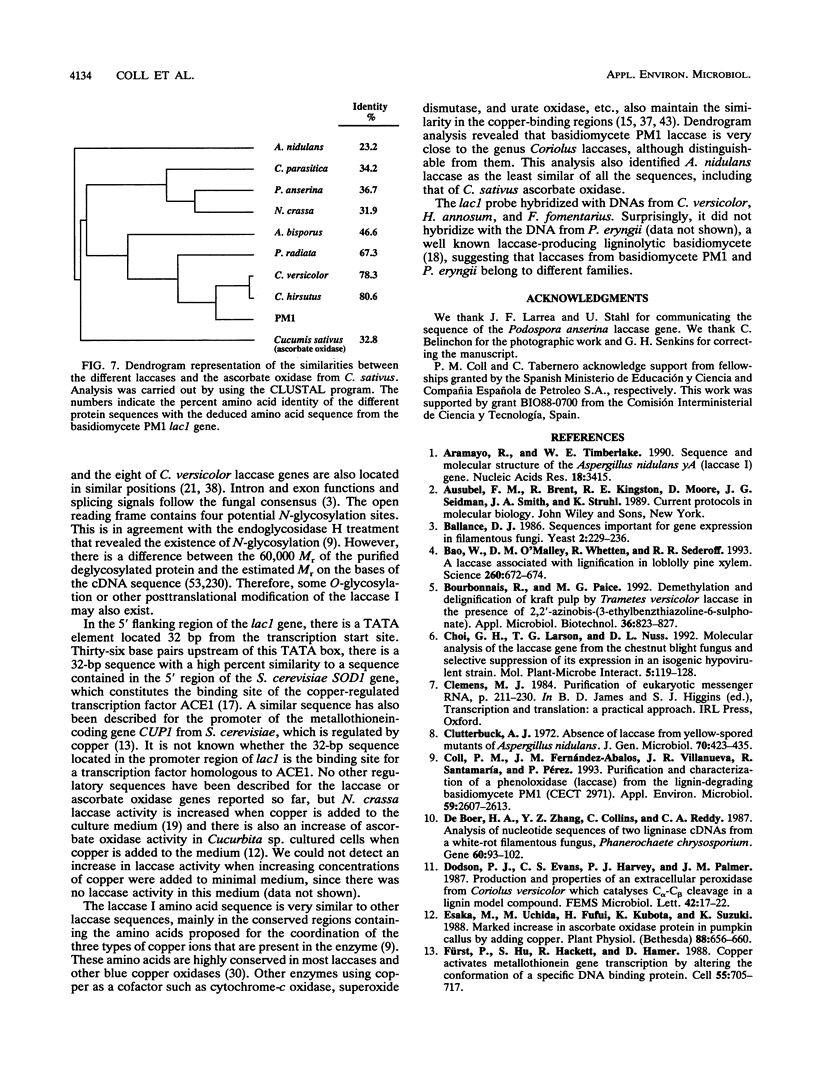
We have isolated and characterized the cDNA and genomic DNA coding for a phenoloxidase, laccase I, previously purified from culture supernatant of the newly isolated ligninolytic basidiomycete PM1 (CECT 2971). A cDNA library from basidiomycete PM1 was constructed, and laccase-encoding cDNAs were identified by screening with antiserum raised against the purified enzyme. The lac1 gene coding for the laccase was identified in a partial genomic library by using the isolated cDNA as a probe. Nucleotide sequence determination of the full-length cDNA revealed an open reading frame of 1,551 bp encoding a polypeptide of 517 amino acid residues with a putative signal peptide of 21 amino acid residues. Ten small introns interrupted the genomic DNA. A single 1.8-kb transcript mRNA was detected by Northern (RNA) blot analysis, and its 5' end maps to a position 51 bp upstream from the site of initiation of protein synthesis. Eukaryotic regulatory sequences, CAAT and TATA, were observed in the 5' flanking region, which also contains sequences similar to those of copper-regulated proteins. Comparative analysis of the predicted amino acid sequence showed that basidiomycete PM1 laccase I had great similarity to the laccases from Coriolus versicolor, Coriolus hirsutus, and Phlebia radiata.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aramayo R., Timberlake W. E. Sequence and molecular structure of the Aspergillus nidulans yA (laccase I) gene. Nucleic Acids Res. 1990 Jun 11;18(11):3415–3415. doi: 10.1093/nar/18.11.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballance D. J. Sequences important for gene expression in filamentous fungi. Yeast. 1986 Dec;2(4):229–236. doi: 10.1002/yea.320020404. [DOI] [PubMed] [Google Scholar]
- Bao W., O'malley D. M., Whetten R., Sederoff R. R. A laccase associated with lignification in loblolly pine xylem. Science. 1993 Apr 30;260(5108):672–674. doi: 10.1126/science.260.5108.672. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Larson T. G., Nuss D. L. Molecular analysis of the laccase gene from the chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- Clutterbuck A. J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J Gen Microbiol. 1972 May;70(3):423–435. doi: 10.1099/00221287-70-3-423. [DOI] [PubMed] [Google Scholar]
- Coll P. M., Fernández-Abalos J. M., Villanueva J. R., Santamaría R., Pérez P. Purification and characterization of a phenoloxidase (laccase) from the lignin-degrading basidiomycete PM1 (CECT 2971). Appl Environ Microbiol. 1993 Aug;59(8):2607–2613. doi: 10.1128/aem.59.8.2607-2613.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaka M., Uchida M., Fukui H., Kubota K., Suzuki K. Marked increase in ascorbate oxidase protein in pumpkin callus by adding copper. Plant Physiol. 1988 Nov;88(3):656–660. doi: 10.1104/pp.88.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Germann U. A., Lerch K. Isolation and partial nucleotide sequence of the laccase gene from Neurospora crassa: amino acid sequence homology of the protein to human ceruloplasmin. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8854–8858. doi: 10.1073/pnas.83.23.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann U. A., Müller G., Hunziker P. E., Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J Biol Chem. 1988 Jan 15;263(2):885–896. [PubMed] [Google Scholar]
- Gralla E. B., Thiele D. J., Silar P., Valentine J. S. ACE1, a copper-dependent transcription factor, activates expression of the yeast copper, zinc superoxide dismutase gene. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8558–8562. doi: 10.1073/pnas.88.19.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., Lerch K. The influence of copper on the induction of tyrosinase and laccase in Neurospora crassa. FEBS Lett. 1987 Jul 27;219(2):335–338. doi: 10.1016/0014-5793(87)80247-8. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Losick R. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J Mol Biol. 1986 Oct 20;191(4):615–624. doi: 10.1016/0022-2836(86)90449-3. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kojima Y., Tsukuda Y., Kawai Y., Tsukamoto A., Sugiura J., Sakaino M., Kita Y. Cloning, sequence analysis, and expression of ligninolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J Biol Chem. 1990 Sep 5;265(25):15224–15230. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis N. G., Yamamoto E. Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships. Eur J Biochem. 1990 Jan 26;187(2):341–352. doi: 10.1111/j.1432-1033.1990.tb15311.x. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ohkawa J., Okada N., Shinmyo A., Takano M. Primary structure of cucumber (Cucumis sativus) ascorbate oxidase deduced from cDNA sequence: homology with blue copper proteins and tissue-specific expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1239–1243. doi: 10.1073/pnas.86.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. R., Smith M., Britnell C. H., Wood D. A., Thurston C. F. Identification of two laccase genes in the cultivated mushroom Agaricus bisporus. J Gen Microbiol. 1993 Jun;139(Pt 6):1209–1218. doi: 10.1099/00221287-139-6-1209. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saloheimo M., Niku-Paavola M. L., Knowles J. K. Isolation and structural analysis of the laccase gene from the lignin-degrading fungus Phlebia radiata. J Gen Microbiol. 1991 Jul;137(7):1537–1544. doi: 10.1099/00221287-137-7-1537. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. W., Lee C. C., Muzny D. M., Caskey C. T. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9412–9416. doi: 10.1073/pnas.86.23.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Zhang Y. Z., Collins C., Reddy C. A. Analysis of nucleotide sequences of two ligninase cDNAs from a white-rot filamentous fungus, Phanerochaete chrysosporium. Gene. 1987;60(1):93–102. doi: 10.1016/0378-1119(87)90217-4. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]