Abstract
Many aspects of physiology and behavior follow a circadian rhythm. Brain and muscle Arnt-like protein-1 (BMAL1) is a key component of the mammalian molecular clock, which controls circadian oscillations. In the rat, the gene encoding Bmal1 is located within hypertension susceptibility loci. We analyzed the SNP distribution pattern in a congenic interval associated with hypertension in the spontaneously hypertensive rat (SHR), and we show that Bmal1 maps close to a region genetically divergent between SHR and its normotensive (Wistar–Kyoto) counterpart. Bmal1 sequencing in rat strains identified 19 polymorphisms, including an SHR promoter variant that significantly affects Gata-4 activation of transcription in transient transfection experiments. A genetic association study designed to test the relevance of these findings in 1,304 individuals from 424 families primarily selected for type 2 diabetes showed that two BMAL1 haplotypes are associated with type 2 diabetes and hypertension. This comparative genetics finding translated from mouse and rat models to human provides evidence of a causative role of Bmal1 variants in pathological components of the metabolic syndrome.
Keywords: comparative genomics, genetic polymorphism, molecular clock, SNP, sequence variation
The regulation of a wide range of biological functions in mammals is subject to circadian variation controlled by the master circadian pacemaker in the suprachiasmatic nucleus of the hypothalamus (1, 2). It has long been observed that pathologic cardiac events display diurnal rhythm patterns. The occurrence of acute myocardial infarction, myocardial ischemia, sudden cardiac death, and stroke shows a markedly increased incidence from dawn to noon (3–5). Blood pressure usually exhibits a circadian pattern of a nightly decrease, and loss of this nocturnal drop in blood pressure (“non-dipper” pattern) correlates with a higher risk of cardiovascular complications (6, 7).
Growing evidence supports the existence of a functional relationship between systems regulating the circadian clock and cardiovascular physiology and pathology in humans and in hypertensive models (8). The circadian expression of plasminogen activator inhibitor-1, a clock-regulated gene that participates in the regulation of thrombolysis and are associated with increased risk of myocardial infarction (9), is compromised in the heart of SHR (10). Although circadian oscillations of the expression of genes of the renin-angiotensin system are conserved in cardiac tissue of SHR, their expression are up-regulated in SHR when compared with normotensive Wistar–Kyoto (WKY) rats (11). Abnormal circadian blood pressure rhythm is associated with hypertensive target-organ damage in SHR stroke-prone rats (12).
The brain and muscle Arnt-like protein-1 (BMAL1) is a core component of the circadian clock and a vital element of the central circadian pacemaker. It is expressed at high level in some regions of the brain (13, 14), and synchronized peripheral oscillation is observed in muscle, lung, kidney, liver, and heart (15). Mice homozygous for Bmal1 deletion exhibit an immediate and complete loss of circadian rhythmicity in constant darkness (16). Inactivation of Bmal1 in mice is associated with a wide range of phenotypes, including decreased body weight; shortened life span; increased sleep time and sleep fragmentation; and altered regulation of blood pressure, glucose homeostasis, lipid metabolism, and adipogenesis (16–19). Changes in Bmal1 circadian expression have been reported in hypertensive models (10). Therefore Bmal1 has an important role in a variety of functions other than regulating circadian rhythm, and its role depends on the tissue type in which it is expressed.
The gene encoding BMAL1 maps to human chromosome 11p15.2. In rat, it is located in a region of chromosome 1q34 harboring quantitative trait loci (QTL) for blood pressure, type 2 diabetes (T2D) mellitus, body weight, cardiac mass, and kidney mass. Compelling evidence supports the existence of several genes accounting for these blood pressure QTL in hypertensive rat strains (20–24). Recent work in a congenic strain (Sisa1a) designed to dissect one of these QTL has identified a 3-Mb region in SHR with significant impact on blood pressure (25). The gene encoding Spondin 1, which maps 0.5 Mb distal to Bmal1 in the congenic interval, was proposed as a candidate (25), but functional mutations accounting for the blood pressure QTL effect remain unknown.
In the present study, we mapped Bmal1 with respect to rat single nucleotide polymorphism (SNP) data in the Sisa1a congenic region and identified functional variants in Bmal1 promoter in WKY and SHR that alter transcription regulation. Genetic studies designed to test the relevance of BMAL1 sequence variants in the etiology of human hypertension identified haplotypes of SNPs in the gene associated with hypertension and T2D.
Results
Genotype Analysis of the Blood Pressure QTL in the Sisa1a Strain.
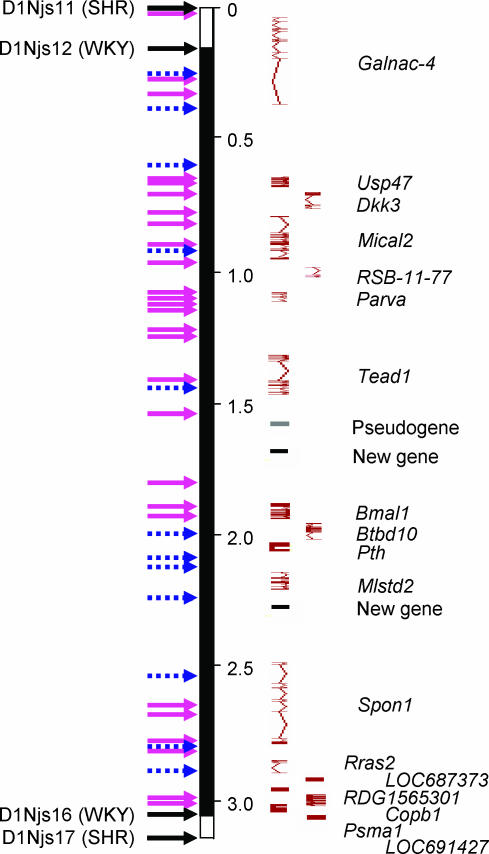
The SHR and WKY strains, which derive from the same outbred stock (Wistar), are likely to share genetic similarities outside hypertension susceptibility loci as the result of generations of breeding of rats selected for high (SHR) or normal blood pressure (WKY). To investigate the genetic relationship between these strains in the Sisa1a congenic region (25), we selected 39 SNPs known to map to the locus and previously characterized for allele variation between rat strains (www.snp-star.eu) [Fig. 1 and supporting information (SI) Table 4]. After SNP sequencing in SHR and WKY parental strains of Sisa1a, 12 SNPs (31%) showed evidence of allele variation in this strain combination. This polymorphism rate is consistent with that given by microsatellite markers (26). We identified a genomic region flanked by Bmal1 and Spondin1 containing an apparent cluster of five consecutive variable SNPs between SHR and WKY, suggesting that this locus may have been inherited during the phenotype assisted selection process used to derive the strains. The other seven variable SNPs were interspersed with conserved SNPs and may correspond to genetic variations that occurred because the strains were independently derived.
Fig. 1.
Genotype data in WKY and SHR strains for SNPs localized in the 3.06-Mb genomic region of chromosome 1 (169.25 Mb at marker D1Njs11; 172.31 Mb at marker D1Njs17) associated with changes in blood pressure regulation in the Sisa1a congenic strain (www.snp-star.eu). Arrows indicate the localization of markers flanking the congenic interval (black) and their genotypes in the congenic strain as reported in ref. 25 and SNPs showing allele variations (blue) or no variations (pink) between SHR and WKY strains. Solid bar represents the chromosomal region carrying WKY alleles in the Sisa1a strain, and open bars at the end of the congenic segment are regions of crossover where genotype is unknown. SNP details are given in SI Table 4. Gene representation and location are from Ensembl (www.ensembl.org/Rattus_norvegicus/index.html). Chromosomal locations refer to the most recent rat genome assembly (RGSC 3.4, genebuild, December 2004).
Sequence Analysis of Rat Bmal1 Coding and 5′ Flanking Regions.
Before sequence analysis, computational sequence homology search (BLAST high-throughput genomic sequence) was carried out with the rat Bmal1 cDNA reference sequence NM_024362 to identify the unfinished high-throughput genomic sequence AC098214, which contains all 20 exons of Bmal1. Detailed gene sequence analysis in 12 inbred rat strains identified two promoter variants (16662-T/C and 18477-T/G), one SNP each in the 5′ untranslated region of exon 1 (18814-C/T) and the 3′ untranslated region of exon 20, and seven intronic polymorphisms, including six SNPs (Table 1). Absence of variants in Bmal1 coding region in SHR and WKY confirms the findings in ref. 25. Three SNPs (16662-T, 99716-T, 104261-A) were specific to the BN strain, and two SNPs were specific to the GK stain (115805-A, 116478-C). WKY and SHR strains showed evidence of allele variation for five SNPs (18477-T/G, 18814-C/T, 18958-C/G, 107111-A/G, 111392-C/T) that were conserved in SHR and Lewis strains.
Table 1.
Bmal1 single nucleotide polymorphisms detected in inbred rat strains
| SNP | Region | WKY | SHR/SHRSP | BN/Ox | GK/Ox | Lewis | F344 | SS/Jr |
|---|---|---|---|---|---|---|---|---|
| 16662-T/C | Promoter | C | C | T | C | C | C | C |
| 18477-T/G | Promoter | T | G | T | T | G | T | T |
| 18814-C/T | 5′UTR/Exon 1 | C | T | C | C | T | C | C |
| 18958-C/G | Intron 1 | C | G | C | C | G | C | C |
| 91363-(GA)n | Intron 9 | 26/28/30 | 17 | 26 | 30 | 17 | 31 | 28 |
| 99716-T/C | Intron 11 | C | C | T | C | C | C | C |
| 104261-A/G | Intron 13 | G | G | A | G | G | G | G |
| 107111-A/G | Intron 15 | G | A | A | A | A | G | A |
| 111392-C/T | Intron 17 | C | T | C | T | T | C | T |
| 115805-G/A | Intron 19 | G | G | G | A | G | G | G |
| 116478-T/C | 3′UTR/Exon 20 | T | T | T | C | T | T | T |
WKY strains were from the colonies bred in Heidelberg, Leicester, and Izumo. SHR/SHRSP strains were from the colonies maintained in Heidelberg, Leicester, Izumo, and Glasgow. Number of repeats (91363-(GA)n) in intron 9 varied among WKY colonies bred at Heidelberg (n = 26), Leicester (n = 28), and Izumo (n = 30). SS/Jr, Dahl salt-sensitive hypertensive rat. The number on SNPs refers to the variant position in AC098214 (HTGS 13-Nov-2002).
After transcriptional binding site scan (Tfsitescan program, available at www.ifti.org), we found that the promoter SNP 18477-T/G located at position −240 from initiation start-site (based on rat EST CB579395) alters the GATA or nuclear factor NF-E1 consensus sequences (27). The 18814-C/T SNP located in the exon 1 untranslated region may affect a paired box gene 6 (Pax6) transcriptional regulator element binding domain (28).
Functional Analysis of Rat Bmal1 Promoter Polymorphisms.
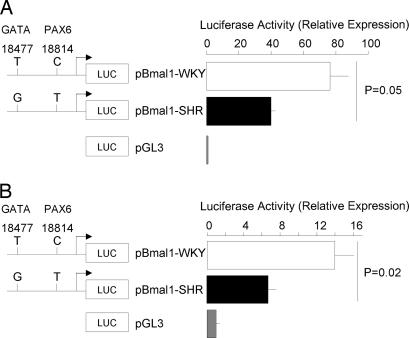
To test the possible effects of Bmal1 promoter SNPs (18477-T/G and 18814-C/T) on GATA- or Pax6-mediated regulation of Bmal1 transcription, the region containing the SNP alleles of the normotensive (WKY) or hypertensive (SHR, SHR stroke-prone) strain was cloned upstream of the firefly luciferase gene to transiently transfect human 293 embryonic kidney (HEK293) cells or hepatoma Hep3B cells (Fig. 2). The promoter variants were constitutively active in both cell types. However, the activity of the WKY promoter was consistently 2-fold higher than that of the SHR promoter. These results indicate that both promoters are functional and that the SNPs 18477-T/G and 18814-C/T alter Bmal1 promoter elements that affect gene expression regulation.
Fig. 2.
Effects of Bmal1 promoter sequence variants on relative luciferase activity of human 293 embryonic kidney (HEK293) or hepatoma (Hep3B) cells. (A) Embryonic kidney (HEK293) cells. (B) Hepatoma (Hep3B) cells. Proliferating cells were transfected with the control vector pGL3-Basic or Bmal1 promoter-luciferase reporter designed to test the effect of promoter SNPs 18477-T/G and 18814-C/T (WKY/SHR allele) on gene transcription (see Materials and Methods). Luciferase reporter gene expression was determined 48 h after transfection. Transfection efficiencies were normalized to lacZ activity of cotransfected pCMX-lacZ plasmid. Results are expressed as the increase in luciferase activity relative to the pGL3-Basic. Results are the mean ± SEM and are representative of four separate experiments performed in duplicate.
Bmal1 Promoter Polymorphisms Affect Gata-4-Stimulated Transcription.
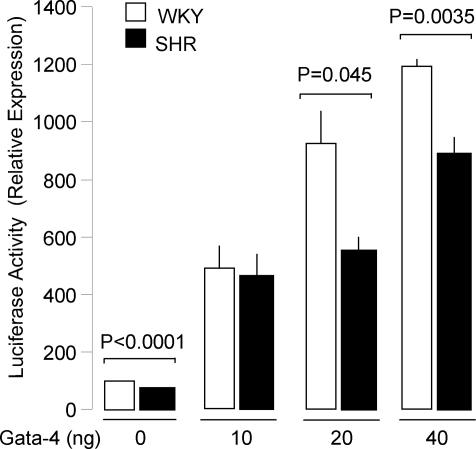
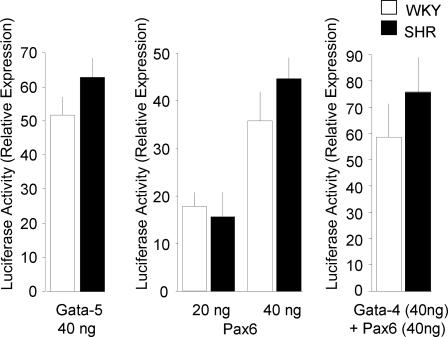
We then assessed the function of Bmal1 promoter polymorphisms on GATA and Pax6-mediated gene transcription, using WKY or SHR promoter reporter plasmids cotransfected in Hep3B cells with plasmids expressing Gata-4, Gata-5, or Pax6 proteins. We show that transfection of the plasmids expressing Gata-4 or Pax6 activate both WKY and SHR Bmal1 promoter constructs in a dose-dependent manner (Figs. 3, 4), indicating that the predicted binding sites to these transcription factors are functional. Transcription activity of Bmal1 promoter constructs was also increased, but to a lesser extent, by Gata-5 (Fig. 4). However, at both 20-ng and 40-ng doses of Gata-4 expressing vector, transcriptional activity of the SHR Bmal1 promoter construct was significantly lower than that of the WKY construct (Fig. 3). In contrast, the levels of Pax6 and Gata-5 stimulated transcription of the WKY and SHR Bmal1 promoter constructs were similar (Fig. 4). The cotransfection of plasmids expressing Gata-4 and Pax6 led to a further enhancement of transcription by both SHR and WKY Bmal1 promoters indicating cooperation between Gata-4 and Pax6 to activate transcription. The cotransfection of Gata-4 and Pax6 also levels the transcriptional activity conferred to the two promoters (Fig. 4).
Fig. 3.
Gata-4-mediated activation of Bmal1 promoter reporter constructs. Hep3B hepatoma cells were cotransfected with Bmal1 promoter constructs containing either the WKY alleles (18477-T and 18814-C) or the SHR alleles (18477-G and 18814-T) and increasing doses of plasmid expressing Gata-4 to determine the maximal activation of Bmal1 promoter. Results are expressed as the increase in luciferase activity relative to the pGL3-Basic. Results are the mean ± SEM and are representative of three separate experiments performed in duplicate.
Fig. 4.
Activation of Bmal1 promoter reporter constructs by Gata-5, Pax6, and Gata-4. Hep3B hepatoma cells were cotransfected with Bmal1 promoter constructs containing either the WKY alleles (18477-T and 18814-C) or the SHR alleles (18477-G and 18814-T) and the indicated expression plasmids. Two concentrations (20 and 40 ng) of Pax6 expressing vector were used. Results are expressed as the increase in luciferase activity relative to the pGL3-Basic. Results are the mean ± SEM and are representative of two separate experiments performed in duplicate.
Overall, these results indicate that Gata-4 and Pax6 activate transcription driven by the Bmal1 promoter and that they can cooperate to activate the Bmal1 promoter. The variant 18814-C/T does not change Pax6-mediated activation of transcription, whereas the variant 18477-T/G significantly reduces the transcriptional activation of the SHR Bmal1 promoter by Gata-4. Finally, Pax6 coexpression with Gata-4 levels these changes of transcription activity.
BMAL1 SNPs Are Associated with Hypertension and T2D in Humans.
To test the relevance of BMAL1 polymorphisms to human hypertension and extend the study to the broad context of insulin resistance, which accounts for the spectrum of SHR pathophysiology (29) and the biological function of the gene (17, 19), we carried out extensive sequence and SNP analyses in British families ascertained through a T2D proband (30). The National Center for Biotechnology Information dbSNP database, the International HapMap Project SNP database, and our original sequencing data in 96 individuals provided us with 19 SNPs for the initial phase of SNP genotyping. A further 40 SNPs were chosen to increase marker density in the region showing provisional association with either hypertension or T2D (see Material and Methods). These markers were genotyped in 1,304 individuals from 424 British T2D families [Diabetes in Families (DIF) study collection]. The location and linkage disequilibrium information for the markers are shown in SI Fig. 5. We used a family-based test of linkage and association (TRANSMIT software) to analyze the data (31). TRANSMIT calculates a score–test statistic that is insensitive to population stratification, appropriately weights affected siblings, allows for missing parental genotypes, and provides a complementary test to the classic transmission disequilibrium test (32).
Single-marker analysis with hypertension and T2D in DIF, using TRANSMIT, is given in SI Table 5. The strongest evidence for association to hypertension was found with rs6486121 (P = 3.6 × 10−3); the strongest evidence for association to T2D was found with rs7950226 (P = 5.4 × 10−3). After correcting for multiple testing (59 SNPs), none of the SNPs were significantly (P ≥ 0.19) associated with either hypertension or T2D in an experiment-wise context. We further examined the data to search for haplotype associations that can be more powerful if multiple variants in a gene influence disease risk. Tables 2 and 3 provide a summary of the haplotype analyses from the DIF study. The strongest evidence for association in this family study was found with the hypertension phenotype (P = 3.3 × 10−4), using a three-marker haplotype (rs6486121, rs3789327, rs969485). A second two-marker haplotype (rs7950226, rs11022775) showed association with T2D (P = 0.002). The SNPs associated with hypertension or T2D were not in the same haplotype block (SI Fig. 5).
Table 2.
TRANSMIT analysis of BMAL1 polymorphisms in the DIF collection
| Disease | First marker | Second marker | Third marker | Step 1 |
Step 2 |
Step 3 |
|||
|---|---|---|---|---|---|---|---|---|---|
| χ 2(df) | P | χ 2(df) | P | χ 2(df) | P | ||||
| Hypertension | rs6486121 | rs3789327 | rs969485 | 8.50 (1) | 3.6 × 10−3 | 17.94 (3) | 4.5 × 10−3 | 27.01 (7) | 3.3 × 10−4 |
| T2D | rs7950226 | rs11022775 | 7.74 (1) | 5.4 × 10−3 | 14.50 (3) | 2.3 × 10−3 | |||
Global P values (i.e., testing overall haplotype–disease association) are shown at each step of the forward-selection procedure. df denotes the degrees of freedom associated with the TRANSMIT χ2 statistic.
Table 3.
Three-locus BMAL1 haplotypes associated with increased disease risk in the DIF collection
| Disease | Haplotype | GRR (CI) | Frequency |
|---|---|---|---|
| Hypertension | CCA* | 2.14 (1.45–3.38) | 0.15 |
| T2D | AC† | 1.54 (1.22–1.97) | 0.44 |
Physical locations are based on dbSNP build 126. GRR, genotypic risk ratio (95% confidence interval).
*Marker order: rs6486121 (13,312,346 bp), rs3789327 (13,341,892 bp), rs969485 (13,359,619 bp).
†Marker order: rs7950226 (13,274,715 bp), rs11022775 (13,330,340 bp).
Discussion
In this article, we describe a series of investigations in the laboratory rat and in a human cohort which provide evidence for a causal relationship between Bmal1 sequence polymorphisms and human hypertension. Genetic studies of blood pressure QTLs in the rat are progressing with the development of congenic models designed to fine map hypertension genes, and advanced knowledge of allele variation between strains (www.snp-star.eu), which can assist candidate gene selection in genetically divergent regions of the genome. Analysis of SHR and WKY SNP alleles at the blood pressure QTL in the Sisa1a congenic strain suggests that the interval flanked by Bmal1 and Spon1 may correspond to an ancestral haplotype containing hypertensive gene(s) originally present in outbred Wistar rats and selected in SHR. The observation of an apparent complete genetic conservation between SHR and WKY for 170 consecutive markers >16.6 Mb of this QTL in the congenic strain WISA1 (10.7 cM, 29.2 Mb) distal to the Sisa1a region (33) supports the applicability of this strategy to select positional candidate genes (data not shown).
Our findings contribute to the characterization of functional regulatory elements in the promoter of Bmal1. The promoter variant 18477-T/G in SHR and WKY appears to disrupt a transcription factor binding site for GATA. The SHR allele (18477-G) is associated with a marked reduction of transcription efficiency and a significant impairment of Gata-4-mediated transcription activation that the overexpression of Pax6 compensates. Because GATA-4 is known to be expressed in cells of the cardiovascular system (34, 35), this promoter polymorphism may induce changes in Bmal1 expression in key organs involved in blood pressure regulation. In addition, as Pax6 expression is positively regulated by Clock and Bmal1 (36), reduced Bmal1 transcription in SHR may result in decreased expression of Pax6, which may in turn fail to compensate GATA-4-mediated Bmal1 expression down-regulation and directly affect glucose homeostasis (17, 37). We have verified the full sequence conservation in SHR and WKY of other components of the molecular clock, Per1 (data not shown) and Clock (38), which are unlikely to alter allele specific biological effects of Bmal1 variants.
The in vivo consequences of SHR Bmal1 promoter variants and their involvement in blood pressure regulation remain to be established. The Sisa1a congenic strain provides a highly practical tool for a wide range of in vivo investigations relevant to Bmal1 biological functions (17, 19) and for testing possible relationships between hypertension and changes in the circadian oscillations of blood pressure (6). Altered levels of Bmal1 may contribute to hypertension in SHR through aberrant sleep pattern and changes in the macrostructure of sleep, which have been documented in SHR (39) and Bmal1−/− mice (40), and are similar to disruptions reported in hypertensive patients (41). A causative link between obstructive sleep apnea and hypertension exists in humans (42, 43).
The detection of Bmal1 functional SNPs in an SHR-derived congenic strain exhibiting altered blood pressure regulation raises fundamental questions in quantitative genetics. It is possible that Bmal1 SNPs were serendipitously isolated during the selection of the SHR strain simply as a consequence of their physical linkage to a hypertension-causing gene. It is equally possible that Bmal1 variants have been selected in SHR for their roles on the regulation of a broad range of biological processes, including blood pressure (19), and therefore directly underlie hypertension QTL effects. This hypothesis is supported by the fact that the SHR, which is primarily a model of essential hypertension caused by selected naturally occurring variants, is widely accepted as a model of genetically determined metabolic syndrome (29). The relationship between alteration of components of the circadian clock and pathophysiological components of the metabolic syndrome has been clearly established in mice (17, 19). Finally, the most significant mutations documented in SHR/SHR stroke-prone affect genes encoding the Cd36 antigen and the inositol polyphosphate phosphatase-like 1 (Inppl1, Ship2), which cause insulin resistance (44, 45). These data strongly support the importance of extensive synchronous in vivo investigations in the Sisa1a strain for testing a possible role of Bmal1 in the regulation of insulin sensitivity and blood pressure, even though other SHR variants in the congenic region may contribute to hypertension.
Results from genome-wide scans for human hypertension have detected linkage to chromosome 11 in the region of BMAL1 (46, 47), which in light of our findings in rats and data in Bmal1−/− mice, support BMAL1 as a plausible candidate gene for hypertension. Recent genome-wide association studies identified evidence of associations between BMAL1 SNPs and hypertension (P = 0.0042 for marker rs9633835) or T2D (minimum P = 0.0036 for marker rs7947951) (SI Table 6) (48, 49). We did not replicate the association with these markers in DIF (P > 0.5).
In summary, we have found a promoter polymorphism in the gene encoding Bmal1 in the SHR strain, which alters its expression in vitro. Supported by data in Bmal1−/− mice (17, 19), we have translated the results from rat to human, with the finding of a strong association of BMAL1 SNP haplotypes with hypertension and T2D in a family study. Replication studies of our genetic findings in humans in other cohorts, especially in different pathophysiological contexts, will test the extent to which defects in BMAL1 are involved in the etiology of hypertension, T2D, and other components of the insulin resistance syndrome.
Materials and Methods
SNP-Based Genotype Analysis of the Critical Congenic Interval in WKY and SHR Rat Strains.
Rat SNPs mapped to the 3.01-Mb genomic region of chromosome 1 associated with changes in blood pressure regulation in Sisa1a congenic strains (25) were obtained from the STAR project (www.snp-star.eu). Polymorphism data were established in WKY and SHR strains. They were verified by sequencing DNA aliquots of WKY and SHR strains of the Leicester colonies used to derive the congenics. Information for all rat SNPs used in this study are available through Ensembl (www.ensembl.org/Rattus_norvegicus/index.html) and reported in SI Table 4.
Bmal1 Promoter and Full-Length Coding Sequence Analysis and SNP Sequencing.
Oligonucleotides were designed to amplify genomic regions of the Sisa1a congenic interval where rat SNPs were reported. Resequencing was carried out only in the SHR and WKY rats of the Leicester colony. For Bmal1 sequence analyses, an unfinished high-throughput genomic sequence (accession no. AC098214) containing the full-length rat Bmal1 (ArntI) gene was used to design oligonucleotides flanking exons for PCR amplification (SI Table 7). Sequence analysis also covered ≈2.1 kb of the promoter region and 0.78 kb of the 3′ untranslated region. The complete Bmal1 sequence (NM_024362) and promoter region were amplified by PCR and sequenced by using genomic DNA of 12 inbred rat strains described elsewhere (38). The PCR and sequencing products were purified by using Bio-Gel P100 gel (Bio-Rad, Hercules, CA) and Sephadex G50 Superfine (Amersham Biosciences, Uppsala, Sweden) in 96-well Multiscreen-HV filter plates (Millipore, Bedford, MA). Sequencing reactions were performed with BigDye terminators, Version 3.1, and analyzed by ABI PRISM 3700 DNA Analyser (Applied Biosystems, Foster City, CA). Sequence analysis and mutation detection were performed by the Sequence Navigator program (Applied Biosystems, Foster City, CA).
Plasmids.
Plasmids expressing the mouse Gata-4 and Gata-5 were gifts from T. Simon (Washington University School of Medicine, St. Louis, MO). The plasmid expressing the rat Pax6 was a gift from M. Sakai (Hokkaido University Graduate School of Medicine, Sapporo, Japan). pCMX-lacZ was a gift from R. Evans (Salk Institute, San Diego, CA). The fragment of the Bmal1 promoter from SHR, BN, or WKY genomic DNA harboring the SNPs 18477-T/G (−240 bp) and 18814-C/T (+98 bp) was amplified by PCR, using the following primers: 5′-CATCCGCTCGAGTGTGCTTCTGTGCACCAAAT-3′ (forward) and 5′-CATCCGAAGCTTTAAGGGGCGCAGCCTC-3′ (reverse) and cloned into pGL3-basic vector (Promega, Madison, WI) at the XhoI-HindIII sites. Plasmid integrity was verified by sequencing.
Transfection and Luciferase Assays.
These plasmids were used in transient transfection assays. Human 293 embryonic kidney or Hep3B hepatoma cells were plated in 24-well plates at 2.5 × 104 cells per well and transfected the next day, using Fugene 6 (Roche, Burgess Hill, U.K.) according to the manufacturer's instructions. pCMX-lacZ (100 ng) was cotransfected in all experiments, luciferase and lacZ activities were measured as described in ref. 50, and the relative luciferase activity was calculated. Amounts of DNA used per transfection refer to the amounts added per well.
T2D Family Collection.
Informed consent was obtained from all subjects, and the investigation was conducted according to the principles expressed in the Declaration of Helsinki. DIF is a collection of community-based diabetic patients collected in the United Kingdom via their general practitioners (1,304 individuals from 424 families; 41 families had at least one parent available for sampling). These are British families of European descent containing a T2D patient with one or more siblings. Incidence of hypertension in the DIF T2D patients was 38%, whereas 59% of the nondiabetic subjects had hypertension (30). Other phenotype details regarding impaired glycemic control, obesity, and lipid parameters are described in ref. 30.
Human BMAL1 SNP Discovery and Genotyping.
The dbSNP database (www.ncbi.nlm.nih.gov/SNP) and the International HapMap Project SNP database (www.hapmap.org) were used to search for BMAL1 SNPs. Approximately 1.6 kb of the genomic sequence AC022878 containing the human BMAL1 promoter region was resequenced to confirm polymorphism of known SNPs and to discover new ones in a panel of selected U.K. individuals from a hypertension family collection and from the DIF T2D collection (24 hypertensive, 24 nonhypertensive, 24 type 2 diabetics, 24 nondiabetics), using four primer pairs (SI Table 7).
We used a tag-SNP strategy to select 23 SNPs from BMAL1, which captured 100% of alleles with r2 of 0.8 identified by using the HapMap genome browser, based on data from Utah residents of European ancestry. Eighteen of these SNPs were successfully genotyped by the Sequenom iPLEX mass-spec system (Sequenom, San Diego, CA) (see SI Fig. 5 and SI Table 8). Based on the results of an exploratory analysis, further variants were selected in regions that showed preliminary evidence of association with hypertension or T2D (Chr11:13275007–13359711), and assays were designed for 49 further SNPs. Locations of the SNPs with respect to BMAL1 promoter, introns, and exons are given in SI Table 8.
Six markers that had a genotyping call-rate of <80% were eliminated, and the overall success rate was 92.6%. Marker genotypes were tested for Hardy–Weinberg equilibrium, and a χ2 threshold of 9 was used to remove markers that were significantly out of equilibrium, leaving 59 SNPs for association studies.
Statistical Analysis.
Statistical analysis of gene expression data was performed by using unpaired Student's t test. A value of P < 0.05 was considered statistically significant.
For human SNP analyses, standard methods were used to test Hardy–Weinberg equilibrium of genotype frequencies. TRANSMIT software (31) was used to apply a family-based test of linkage and association. TRANSMIT implements both allele and haplotype tests of transmission disequilibrium. TRANSMIT estimates haplotype frequencies in founder, using an EM algorithm so that missing data (incomplete genotypes) can be weighted in the transmission disequilibrium test. A forward-selection strategy was implemented to build a haplotype that is linked/associated with susceptibility to T2D or hypertension Extension of a haplotype was allowed when the overall (“global”) significance of the gene-association was increased, based on the asymptotic P value; this takes into account the increasing number of degrees of freedom with longer haplotypes.
Supplementary Material
Acknowledgments
We thank Theodore Simon and Masaharu Sakai for generously providing reagents, Mark I. McCarthy for providing DIF DNA samples, and Garret A. FitzGerald for helpful discussions which initiated this study. This work was supported by Wellcome Trust Senior in Basic Biomedical Science Fellowship 057733 (to D.G.), Wellcome Cardiovascular Functional Genomics Initiative 066780/Z/01/Z, and European Commission STAR Grant LSHG-CT-2004-005235. The U.K. collection of diabetic families (DIF) was developed by the late Professor R. Turner. We thank Wellcome Trust Case Control Consortium 076113 for genome-wide association genotyping data.
Abbreviations
- CDS
coding sequences
- DIF
Diabetes in Families
- QTL
quantitative trait locus
- SHR
spontaneously hypertensive rat
- T2D
type 2 diabetes
- TDT
transmission disequilibrium test
- WKY
Wistar–Kyoto.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703247104/DC1.
References
- 1.Hastings MH, Reddy AB, Maywood ES. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 2.Moore RY. Annu Rev Med. 1997;48:253–266. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- 3.Maemura K, Layne MD, Watanabe M, Perrell MA, Nagai R, Lee ME. Ann N Y Acad Sci. 2001;947:398–402. doi: 10.1111/j.1749-6632.2001.tb03972.x. [DOI] [PubMed] [Google Scholar]
- 4.Muller JE, Tofler GH, Stone PH. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 5.Portman MA. Circ Res. 2001;89:1084–1086. [PubMed] [Google Scholar]
- 6.Lemmer B. Pharmacol Ther. 2006;111:629–651. doi: 10.1016/j.pharmthera.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 8.Young ME. Am J Physiol Heart Circ Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 9.Hamsten A, de Faire U, Walldius G, Dahlen G, Szamosi A, Landou C, Blomback M, Wiman B. Lancet. 1987;2:3–9. doi: 10.1016/s0140-6736(87)93050-9. [DOI] [PubMed] [Google Scholar]
- 10.Naito Y, Tsujino T, Kawasaki D, Okumura T, Morimoto S, Masai M, Sakoda T, Fujioka Y, Ohyanagi M, Iwasaki T. J Hypertens. 2003;21:1107–1115. doi: 10.1097/00004872-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Naito Y, Tsujino T, Fujioka Y, Ohyanagi M, Iwasaki T. Hypertension. 2002;40:827–833. doi: 10.1161/01.hyp.0000039960.66987.89. [DOI] [PubMed] [Google Scholar]
- 12.Shimamura T, Nakajima M, Iwasaki T, Hayasaki Y, Yonetani Y, Iwaki K. J Hypertens. 1999;17:211–220. doi: 10.1097/00004872-199917020-00005. [DOI] [PubMed] [Google Scholar]
- 13.Honma S, Ikeda M, Abe H, Tanahashi Y, Namihira M, Honma K, Nomura M. Biochem Biophys Res Commun. 1998;250(1):83–87. doi: 10.1006/bbrc.1998.9275. [DOI] [PubMed] [Google Scholar]
- 14.Namihira M, Honma S, Abe H, Tanahashi Y, Ikeda M, Honma K. Neurosci Lett. 1999;267:69–72. doi: 10.1016/s0304-3940(99)00324-9. [DOI] [PubMed] [Google Scholar]
- 15.Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Biochem Biophys Res Commun. 1998;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- 16.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, FitzGerald GA. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Proc Natl Acad Sci USA. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, FitzGerald GA. Proc Natl Acad Sci USA. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwai N, Inagami T. J Hypertens. 1992;10:1155–1157. doi: 10.1097/00004872-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Frantz SA, Kaiser M, Gardiner SM, Gauguier D, Vincent M, Thompson JR, Bennett T, Samani NJ. Hypertension. 1998;32:647–648. doi: 10.1161/01.hyp.32.4.639. [DOI] [PubMed] [Google Scholar]
- 22.Hubner N, Lee Y-A, Lindpaintner K, Ganten D, Kreutz R. Hypertension. 1999;34:643–648. doi: 10.1161/01.hyp.34.4.643. [DOI] [PubMed] [Google Scholar]
- 23.Saad Y, Garrett MR, Lee SJ, Dene H, Rapp JP. Physiol Genomics. 1999;1:119–125. doi: 10.1152/physiolgenomics.1999.1.3.119. [DOI] [PubMed] [Google Scholar]
- 24.St Lezin E, Liu WZ, Wang JM, Wang N, Kren V, Krenova D, Musilova A, Zdobinska M, Zidek V, Lau D, et al. Hypertension. 1997;30:854–858. doi: 10.1161/01.hyp.30.4.854. [DOI] [PubMed] [Google Scholar]
- 25.Clemitson JR, Dixon RJ, Haines S, Bingham AJ, Patel BR, Hall L, Lo M, Sassard J, Charchar FJ, Samani NJ. Circ Res. 2007;100:992–999. doi: 10.1161/01.RES.0000261961.41889.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bihoreau MT, Gauguier D, Kato N, Hyne G, Lindpaintner K, Rapp JP, James MR, Lathrop GM. Genome Res. 1997;7:434–440. doi: 10.1101/gr.7.5.434. [DOI] [PubMed] [Google Scholar]
- 27.Reddy PM, Shen CK. Proc Natl Acad Sci USA. 1991;88:8676–8680. doi: 10.1073/pnas.88.19.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein J, Cai J, Glaser T, Jepeal L, Maas R. J Biol Chem. 1994;269:8355–8361. [PubMed] [Google Scholar]
- 29.Pravenec M, Zidek V, Landa V, Simakova M, Mlejnek P, Kazdova L, Bila V, Krenova D, Kren V. Physiol Res. 2004;53(Suppl 1):S15–S22. [PubMed] [Google Scholar]
- 30.Kaisaki PJ, Delepine M, Woon PY, Sebag-Montefiore L, Wilder SP, Menzel S, Vionnet N, Marion E, Riveline JP, Charpentier G, et al. Diabetes. 2004;53:1900–1904. doi: 10.2337/diabetes.53.7.1900. [DOI] [PubMed] [Google Scholar]
- 31.Clayton D. Am J Hum Genet. 1999;65:1170–1177. doi: 10.1086/302577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spielman RS, McGinnis RE, Ewens WJ. Am J Hum Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- 33.Frantz S, Clemitson JR, Bihoreau MT, Gauguier D, Samani NJ. Hypertension. 2001;38:216–221. doi: 10.1161/01.hyp.38.2.216. [DOI] [PubMed] [Google Scholar]
- 34.Molkentin JD. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki YJ, Day RM, Tan CC, Sandven TH, Liang Q, Molkentin JD, Fanburg BL. J Biol Chem. 2003;278:17525–17531. doi: 10.1074/jbc.M210465200. [DOI] [PubMed] [Google Scholar]
- 36.Morgan R. Dev Dyn. 2004;230:643–650. doi: 10.1002/dvdy.20097. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda T, Kajimoto Y, Fujitani Y, Watada H, Yamamoto S, Watarai T, Umayahara Y, Matsuhisa M, Gorogawa S, Kuwayama Y, et al. Diabetes. 2002;51:224–230. doi: 10.2337/diabetes.51.1.224. [DOI] [PubMed] [Google Scholar]
- 38.Woon PY, Curtis AM, Kaisaki PJ, Argoud K, Wallace KJ, Bihoreau MT, FitzGerald GA, Gauguier D. Genomics. 2006;87:208–217. doi: 10.1016/j.ygeno.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Carley DW, Trbovic S, Radulovacki M. Physiol Behav. 1996;59:827–831. doi: 10.1016/0031-9384(95)02205-8. [DOI] [PubMed] [Google Scholar]
- 40.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 41.Kuo TB, Lai CJ, Shaw FZ, Lai CW, Yang CC. Am J Physiol Heart Circ Physiol. 2004;286:H1170–H1176. doi: 10.1152/ajpheart.00418.2003. [DOI] [PubMed] [Google Scholar]
- 42.Hui DS, To KW, Ko FW, Fok JP, Chan MC, Ngai JC, Tung AH, Ho CW, Tong MW, Szeto CC, et al. Thorax. 2006;61:1083–1090. doi: 10.1136/thx.2006.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson GV, Stradling JR, Davies RJ. Thorax. 2004;59:1089–1094. doi: 10.1136/thx.2003.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, et al. Nat Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 45.Marion E, Kaisaki P, Pouillon V, Gueydan C, Levy J, Bodson A, Krzentowski G, Daubresse J-C, Mockel J, Behrends J, et al. Diabetes. 2002;51:2012–2017. doi: 10.2337/diabetes.51.7.2012. [DOI] [PubMed] [Google Scholar]
- 46.de Lange M, Spector TD, Andrew T. Hypertension. 2004;44:872–877. doi: 10.1161/01.HYP.0000148994.89903.fa. [DOI] [PubMed] [Google Scholar]
- 47.Xu X, Rogus JJ, Terwedow HA, Yang J, Wang Z, Chen C, Niu T, Wang B, Xu H, Weiss S, et al. Am J Hum Genet. 1999;64:1694–1701. doi: 10.1086/302405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, et al. Science. 2007;316:1331–1336. [Google Scholar]
- 49.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, et al. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braganca J, Swingler T, Marques FIR, Jones T, Eloranta JJ, Hurst HC, Shioda T, Bhattacharya S. J Biol Chem. 2002;277:8559–8565. doi: 10.1074/jbc.M110850200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.