Abstract
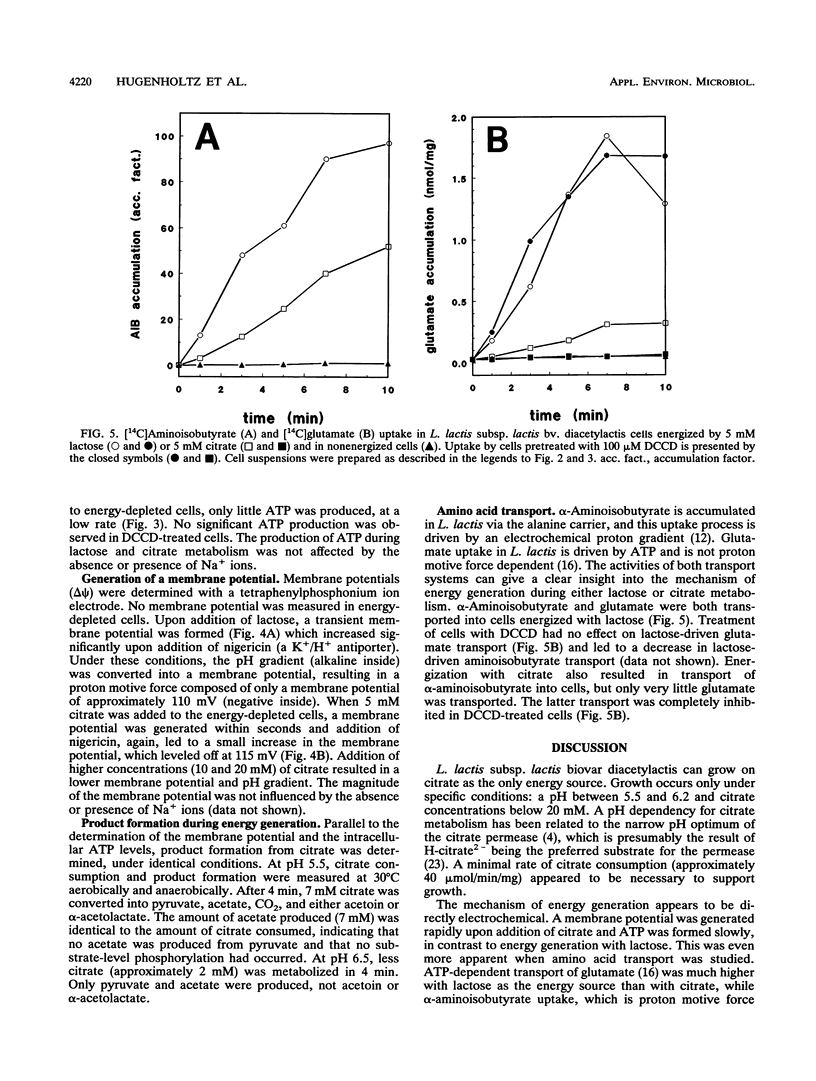
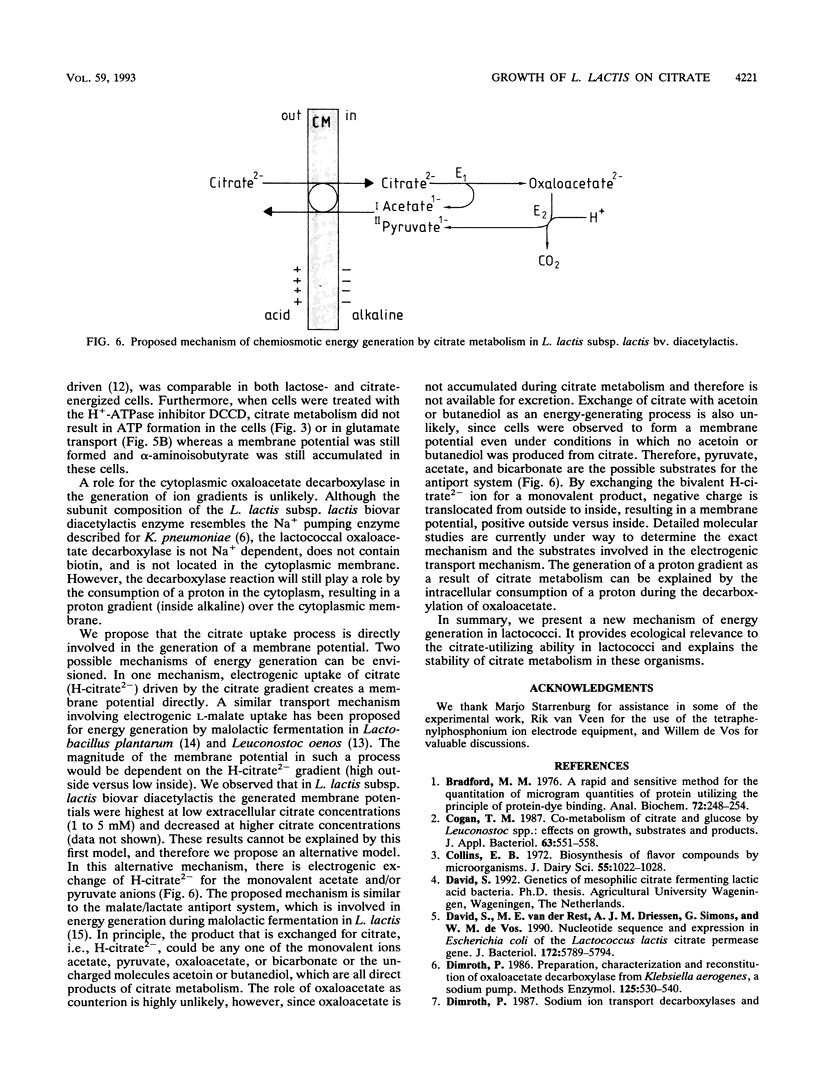
Growth of Lactococcus lactis subsp. lactis biovar diacetylactis was observed on media with citrate as the only energy source. At pH 5.6, steady state was achieved in a chemostat on a citrate-containing medium in the absence of a carbohydrate. Under these conditions, pyruvate, acetate, and some acetoin and butanediol were the main fermentation products. This indicated that energy was conserved in L. lactis subsp. lactis biovar diacetylactis during citrate metabolism and presumably during the conversion of citrate into pyruvate. The presumed energy-conserving step, decarboxylation of oxaloacetate, was studied in detail. Oxaloacetate decarboxylase was purified to homogeneity and characterized. The enzyme has a native molecular mass of approximately 300 kDa and consists of three subunits of 52, 34, and 12 kDa. The enzyme is apparently not sodium dependent and does not contain a biotin moiety, and it seems to be different from the energy-generating oxaloacetate decarboxylase from Klebsiella pneumoniae. Energy-depleted L. lactis subsp. lactis biovar diacetylactis cells generated a membrane potential and a pH gradient immediately upon addition of citrate, whereas ATP formation was slow and limited. In contrast, lactose energization resulted in rapid ATP formation and gradual generation of a proton motive force. These data were confirmed during studies on amino acid uptake. α-Aminoisobutyrate uptake was rapid but glutamate uptake was slow in citrate-energized cells, whereas lactose-energized cells showed the reverse tendency. These data suggest that, in L. lactis subsp. lactis bv. diacetylactis, a proton motive force could be generated during citrate metabolism as a result of electrogenic citrate uptake or citrate/product exchange together with proton consumption by the intracellular oxaloacetate decarboxylase.
Full text
PDF
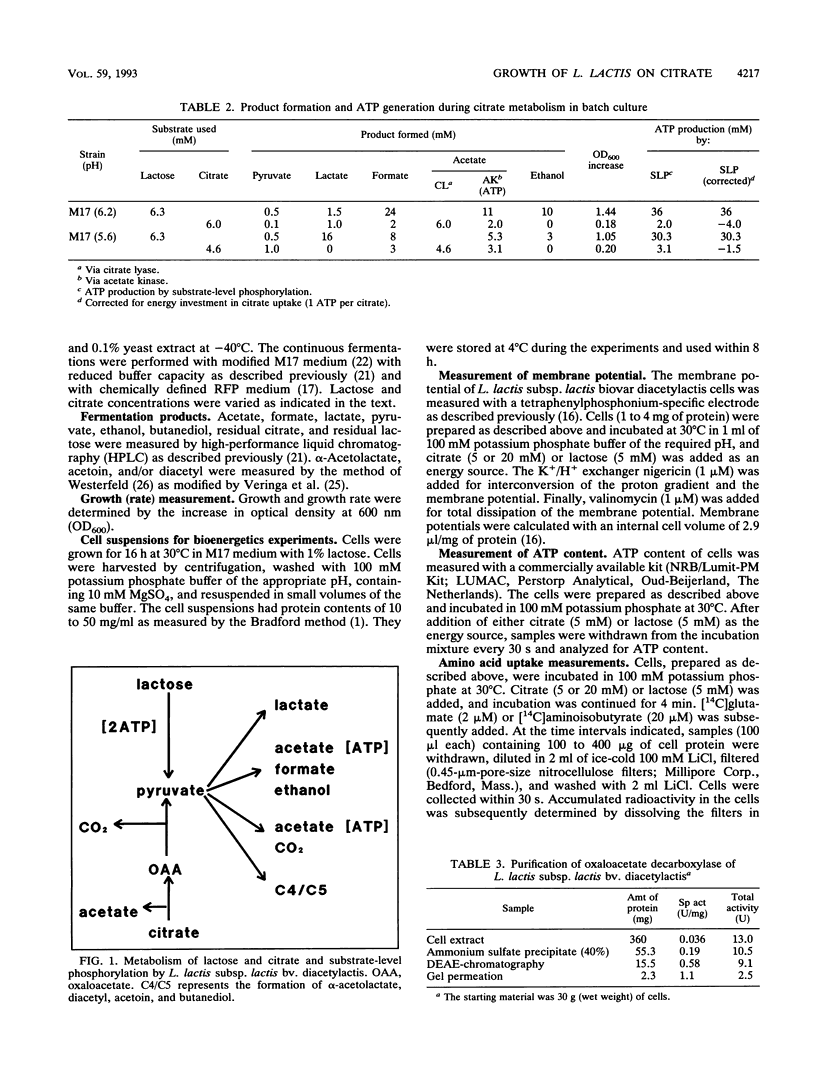
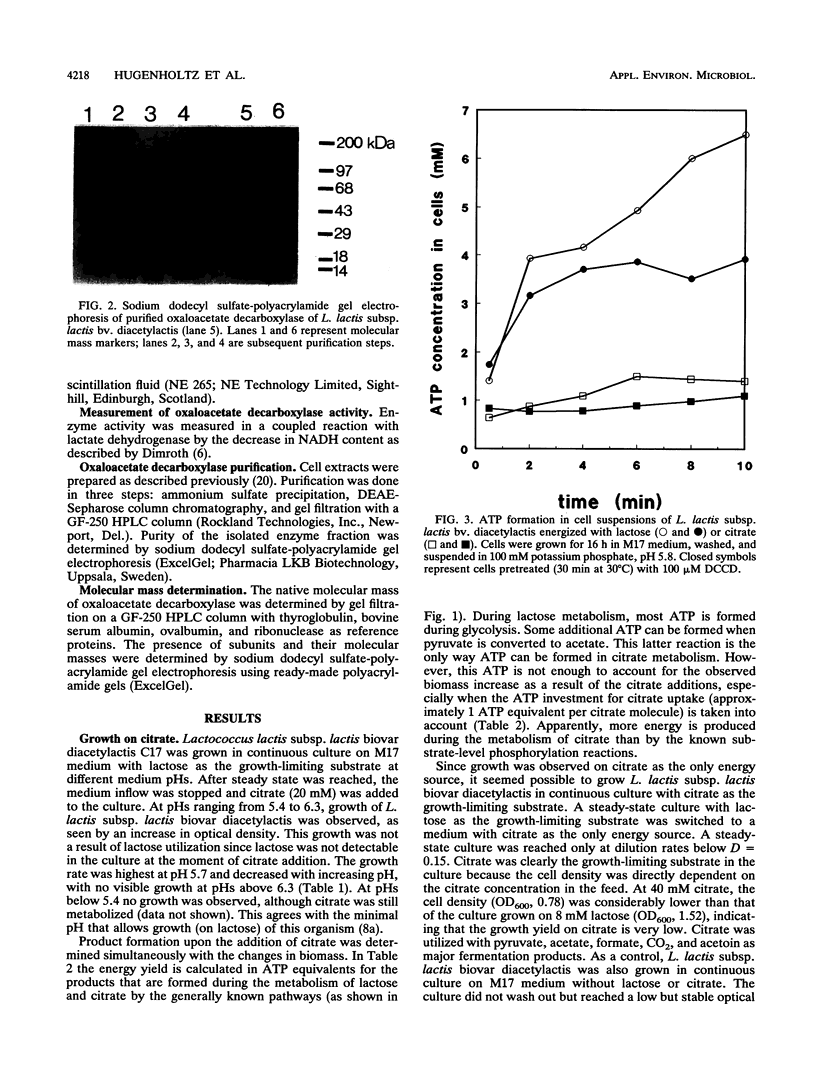
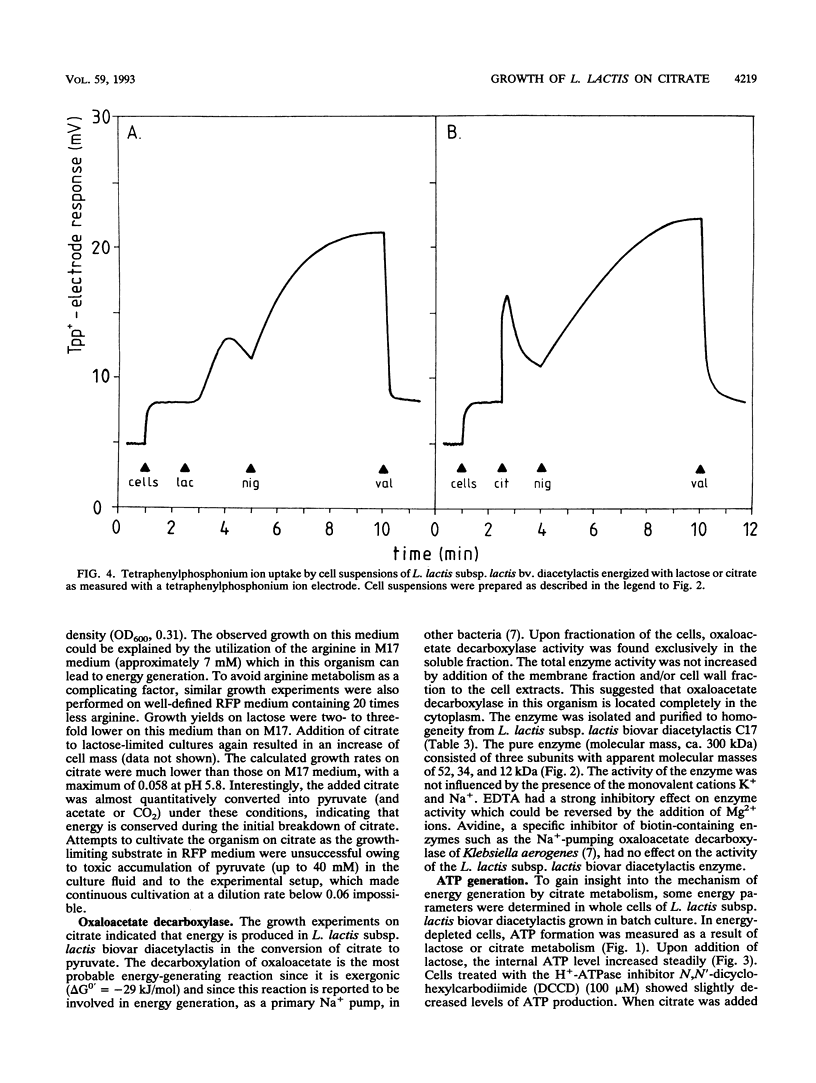

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- David S., van der Rest M. E., Driessen A. J., Simons G., de Vos W. M. Nucleotide sequence and expression in Escherichia coli of the Lactococcus lactis citrate permease gene. J Bacteriol. 1990 Oct;172(10):5789–5794. doi: 10.1128/jb.172.10.5789-5794.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P. Preparation, characterization, and reconstitution of oxaloacetate decarboxylase from Klebsiella aerogenes, a sodium pump. Methods Enzymol. 1986;125:530–540. doi: 10.1016/s0076-6879(86)25042-9. [DOI] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Characterization of Plasmid Deoxyribonucleic Acid in Streptococcus lactis subsp. diacetylactis: Evidence for Plasmid-Linked Citrate Utilization. Appl Environ Microbiol. 1979 Feb;37(2):316–323. doi: 10.1128/aem.37.2.316-323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Otto R. Energy transduction and solute transport in streptococci. Antonie Van Leeuwenhoek. 1983 Sep;49(3):247–257. doi: 10.1007/BF00399501. [DOI] [PubMed] [Google Scholar]
- Loubiere P., Salou P., Leroy M. J., Lindley N. D., Pareilleux A. Electrogenic malate uptake and improved growth energetics of the malolactic bacterium Leuconostoc oenos grown on glucose-malate mixtures. J Bacteriol. 1992 Aug;174(16):5302–5308. doi: 10.1128/jb.174.16.5302-5308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen E. B., Russell J. B., Henick-Kling T. Electrogenic L-malate transport by Lactobacillus plantarum: a basis for energy derivation from malolactic fermentation. J Bacteriol. 1991 Oct;173(19):6199–6206. doi: 10.1128/jb.173.19.6199-6206.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B. Energy transduction in lactic acid bacteria. FEMS Microbiol Rev. 1993 Sep;12(1-3):125–147. doi: 10.1111/j.1574-6976.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., FRANKLIN J. G., PERRY K. D. Correlation of the vitamin requirements with cultural and biochemical characters of Lactobacillus spp. J Gen Microbiol. 1961 Jul;25:473–482. doi: 10.1099/00221287-25-3-473. [DOI] [PubMed] [Google Scholar]
- Snoep J. L., Teixeira de Mattos M. J., Starrenburg M. J., Hugenholtz J. Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and alpha-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol. 1992 Jul;174(14):4838–4841. doi: 10.1128/jb.174.14.4838-4841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrenburg M. J., Hugenholtz J. Citrate Fermentation by Lactococcus and Leuconostoc spp. Appl Environ Microbiol. 1991 Dec;57(12):3535–3540. doi: 10.1128/aem.57.12.3535-3540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Rest M. E., Abee T., Molenaar D., Konings W. N. Mechanism and energetics of a citrate-transport system of Klebsiella pneumoniae. Eur J Biochem. 1991 Jan 1;195(1):71–77. doi: 10.1111/j.1432-1033.1991.tb15677.x. [DOI] [PubMed] [Google Scholar]
- Verhue W. M., Tjan F. S. Study of the Citrate Metabolism of Lactococcus lactis subsp. lactis Biovar Diacetylactis by Means of C Nuclear Magnetic Resonance. Appl Environ Microbiol. 1991 Nov;57(11):3371–3377. doi: 10.1128/aem.57.11.3371-3377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]