Abstract
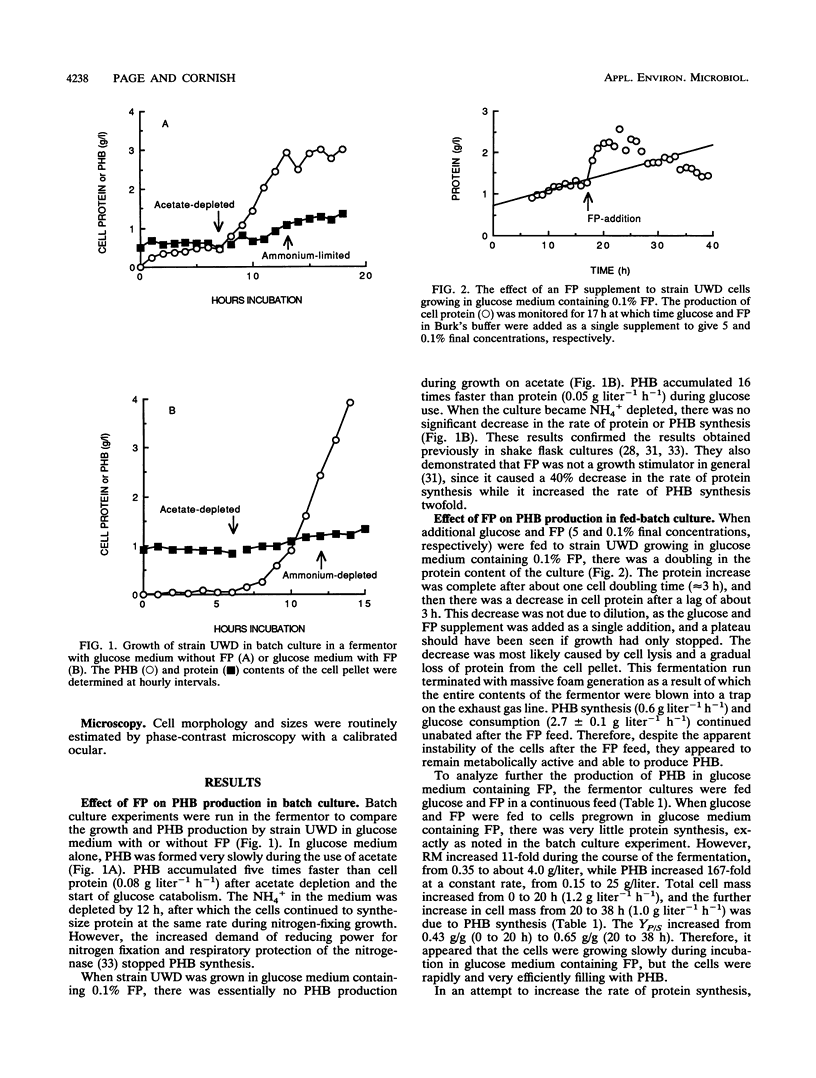
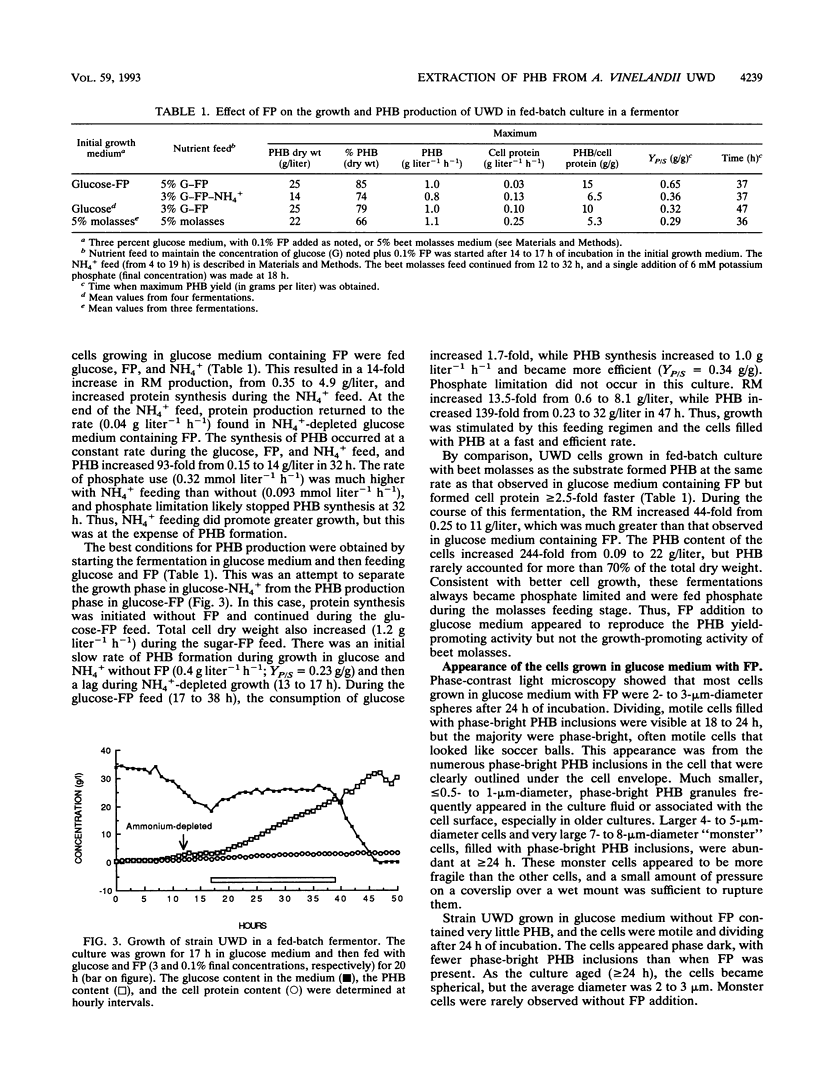
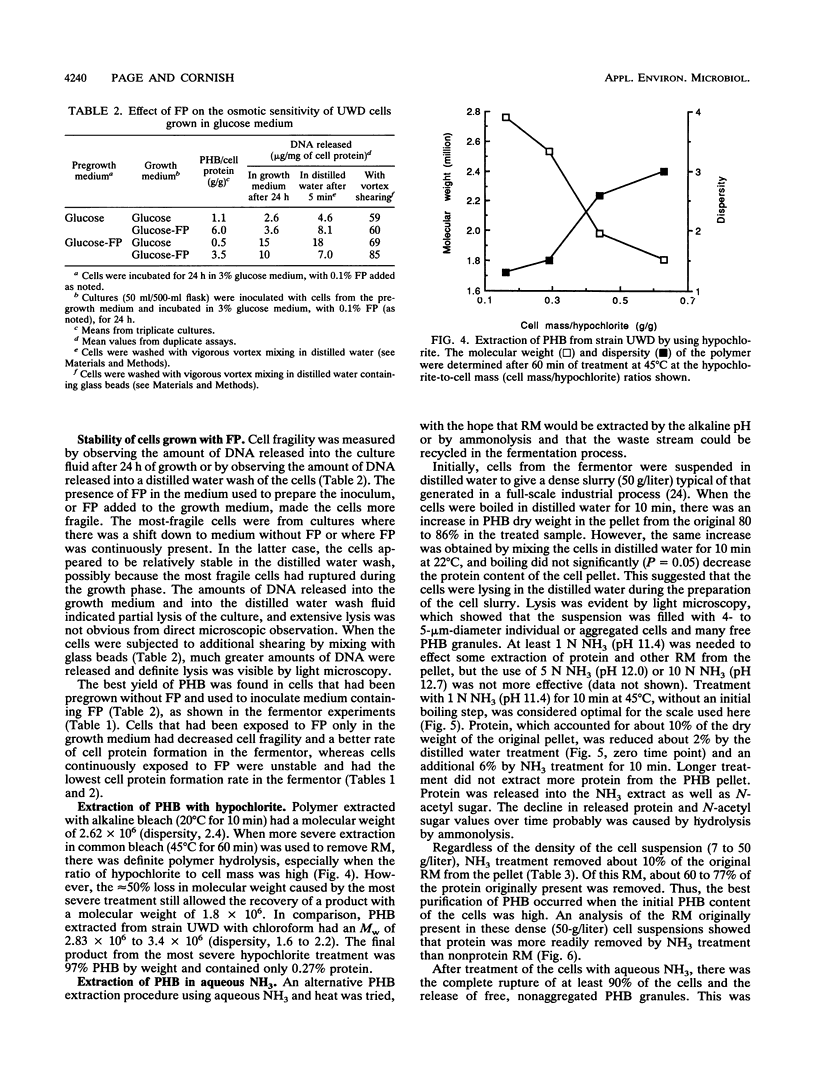
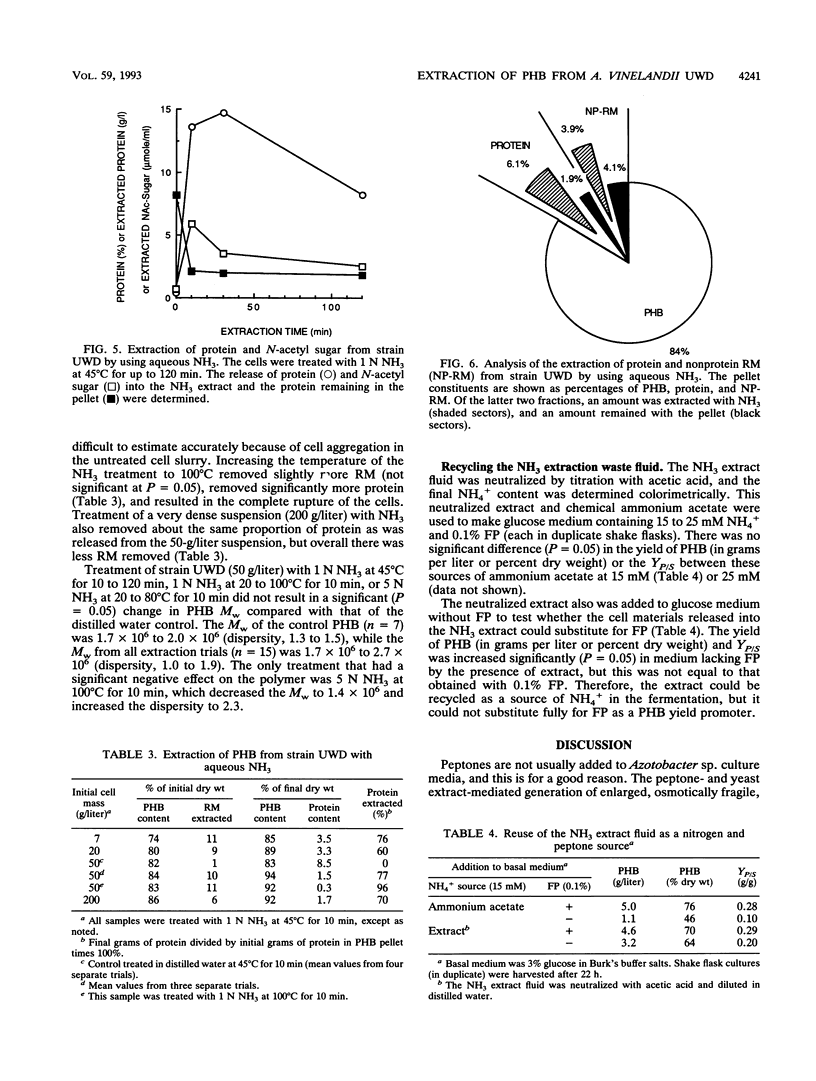
Azotobacter vinelandii UWD was grown in a fermentor with glucose medium with and without 0.1% fish peptone (FP) in batch and fed-batch cultures for the production of the natural bioplastic poly-β-hydroxybutyrate (PHB). Strain UWD formed PHB five times faster than cell protein during growth in glucose and NH4+, but PHB synthesis stopped when NH4+ was depleted and nitrogen fixation started. When FP was added to the same medium, PHB accumulated 16 times faster than cell protein, which in turn was inhibited by 40%, and PHB synthesis was unaffected by NH4+ depletion. Thus, FP appeared to be used as a nitrogen source by these nitrogen-fixing cells, which permitted enhanced PHB synthesis, but it was not a general growth stimulator. The addition of FP to the medium led to the production of large, pleomorphic, osmotically sensitive cells that demonstrated impaired growth and partial lysis, with the leakage of DNA into the culture fluid, but these cells were still able to synthesize PHB at elevated rates and efficiency. When FP was continuously present in fed-batch culture, the yield in grams of polymer per gram of glucose consumed was calculated to range from 0.43 g/g, characteristic of nongrowing cells, to an unprecedented 0.65 g/g. Separation of an FP-free growth phase from an FP-containing growth phase in fed-batch culture resulted in better growth of these pleomorphic cells and good production of PHB (yield, 0.32 g/g). The fragility of these cells was exploited in a simple procedure for the extraction of high-molecular-weight PHB. The cells were treated with 1 N aqueous NH3 (pH 11.4) at 45°C for 10 min. This treatment removed about 10% of the non-PHB mass from the pellet, of which 60 to 77% was protein. The final product consisted of 94% PHB, 2% protein, and 4% nonprotein residual mass. The polymer molecular weight (1.7 × 106 to 2.0 × 106) and dispersity (1.0 to 1.9) were not significantly affected (P = 0.05) by this treatment. In addition, the NH3 extraction waste could be recycled in the fermentation as a nitrogen source, but it did not promote PHB production like FP. A scheme for improved downstream extraction of PHB as well as the merits of using pleomorphic cells in the production of bioplastics is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Dawes E. A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990 Dec;54(4):450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. J., Haywood G. W., Dawes E. A. Biosynthesis and composition of bacterial poly(hydroxyalkanoates). Int J Biol Macromol. 1990 Apr;12(2):102–105. doi: 10.1016/0141-8130(90)90060-n. [DOI] [PubMed] [Google Scholar]
- Bayer-Berger M. M., Ravussin P., Fankhauser H., Freeman J. Effect of three pretreatment techniques on hemodynamic and CSFP responses to skull-pin head-holder application during thiopentone/isoflurane or propofol anesthesia. J Neurosurg Anesthesiol. 1989 Sep;1(3):227–232. doi: 10.1097/00008506-198909000-00004. [DOI] [PubMed] [Google Scholar]
- Bingle W. H. Transformation of Azotobacter vinelandii OP with a broad host range plasmid containing a cloned chromosomal nif-DNA marker. Plasmid. 1988 May;19(3):242–250. doi: 10.1016/0147-619x(88)90042-x. [DOI] [PubMed] [Google Scholar]
- Budwill K., Fedorak P. M., Page W. J. Methanogenic degradation of poly(3-hydroxyalkanoates). Appl Environ Microbiol. 1992 Apr;58(4):1398–1401. doi: 10.1128/aem.58.4.1398-1401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung P. H., Winter A. J. Effects of penicillin and glycine on cell wall glycopeptides of the two varieties of Vibrio fetus. J Bacteriol. 1968 Dec;96(6):1889–1894. doi: 10.1128/jb.96.6.1889-1894.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzier W. D. Materials derived from biomass/biodegradable materials. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):839–842. doi: 10.1073/pnas.89.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maculla E. S., Cowles P. B. The Use of Glycine in the Disruption of Bacterial Cells. Science. 1948 Apr 9;107(2780):376–377. doi: 10.1126/science.107.2780.376. [DOI] [PubMed] [Google Scholar]
- Morgan A. R., Lee J. S., Pulleyblank D. E., Murray N. L., Evans D. H. Review: ethidium fluorescence assays. Part 1. Physicochemical studies. Nucleic Acids Res. 1979 Oct 10;7(3):547–569. doi: 10.1093/nar/7.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Knosp O. Hyperproduction of Poly-beta-Hydroxybutyrate during Exponential Growth of Azotobacter vinelandii UWD. Appl Environ Microbiol. 1989 Jun;55(6):1334–1339. doi: 10.1128/aem.55.6.1334-1339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Manchak J., Rudy B. Formation of poly(hydroxybutyrate-co-hydroxyvalerate) by Azotobacter vinelandii UWD. Appl Environ Microbiol. 1992 Sep;58(9):2866–2873. doi: 10.1128/aem.58.9.2866-2873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Rudy B. An autosampler for fermentation. Biotechniques. 1993 Feb;14(2):228–233. [PubMed] [Google Scholar]
- STROMINGER J. L., BIRGE C. H. NUCLEOTIDE ACCUMULATION INDUCED IN STAPHYLOCOCCUS AUREUS BY GLYCINE. J Bacteriol. 1965 Apr;89:1124–1127. doi: 10.1128/jb.89.4.1124-1127.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROMINGER J. L. Microbial uridine-5'-pyrophosphate N-acetylamino sugar compounds. I. Biology of the penicillin-induced accumulation. J Biol Chem. 1957 Jan;224(1):509–523. [PubMed] [Google Scholar]
- Vela G. R., Rosenthal R. S. Effect of peptone on Azotobacter morphology. J Bacteriol. 1972 Jul;111(1):260–266. doi: 10.1128/jb.111.1.260-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., WILKINSON J. F. The isolation and estimation of the poly-beta-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol. 1958 Aug;19(1):198–209. doi: 10.1099/00221287-19-1-198. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Tsukagoshi N., Miyashiro S., Udaka S. Increased production of alpha-amylase by Bacillus amyloliquefaciens in the presence of glycine. Appl Environ Microbiol. 1983 Jul;46(1):293–295. doi: 10.1128/aem.46.1.293-295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



