Abstract
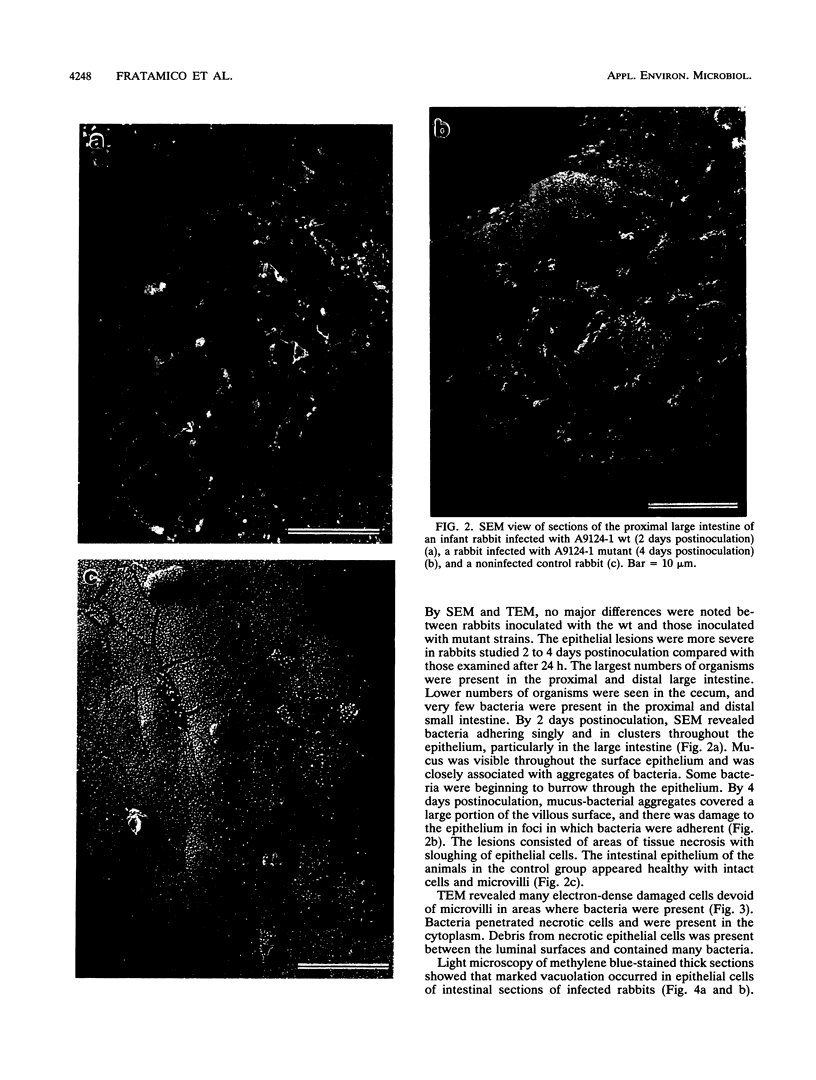
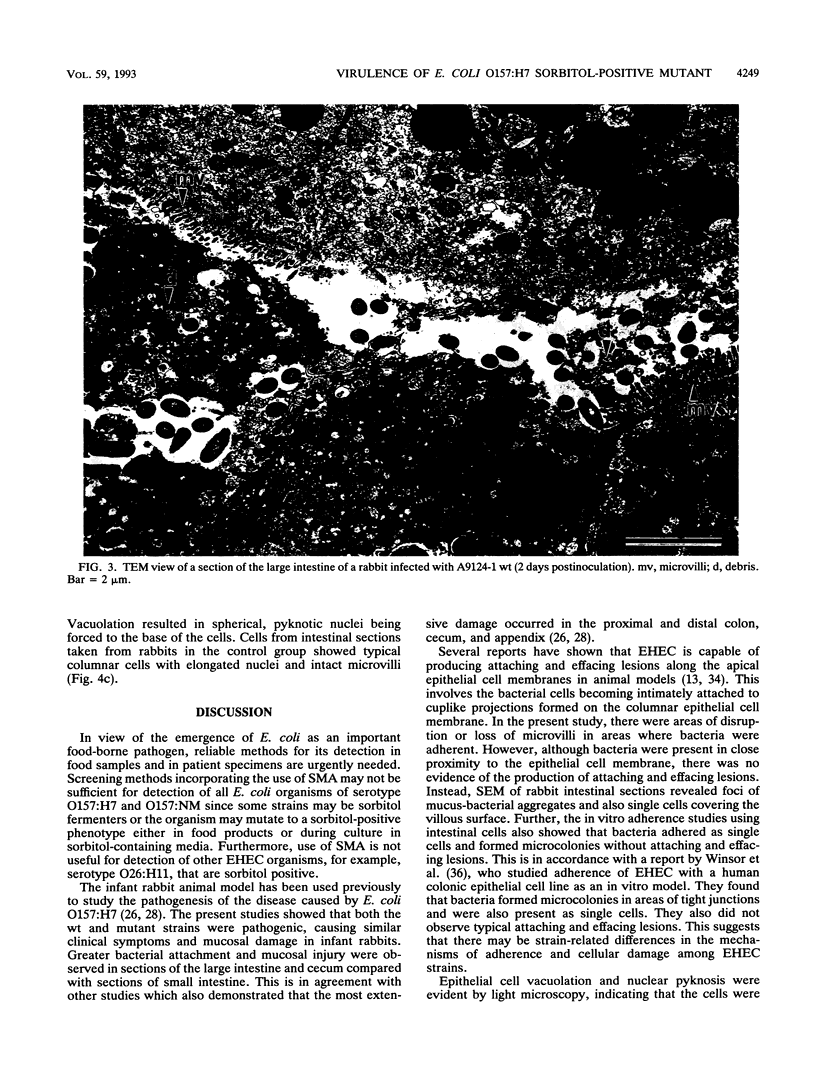
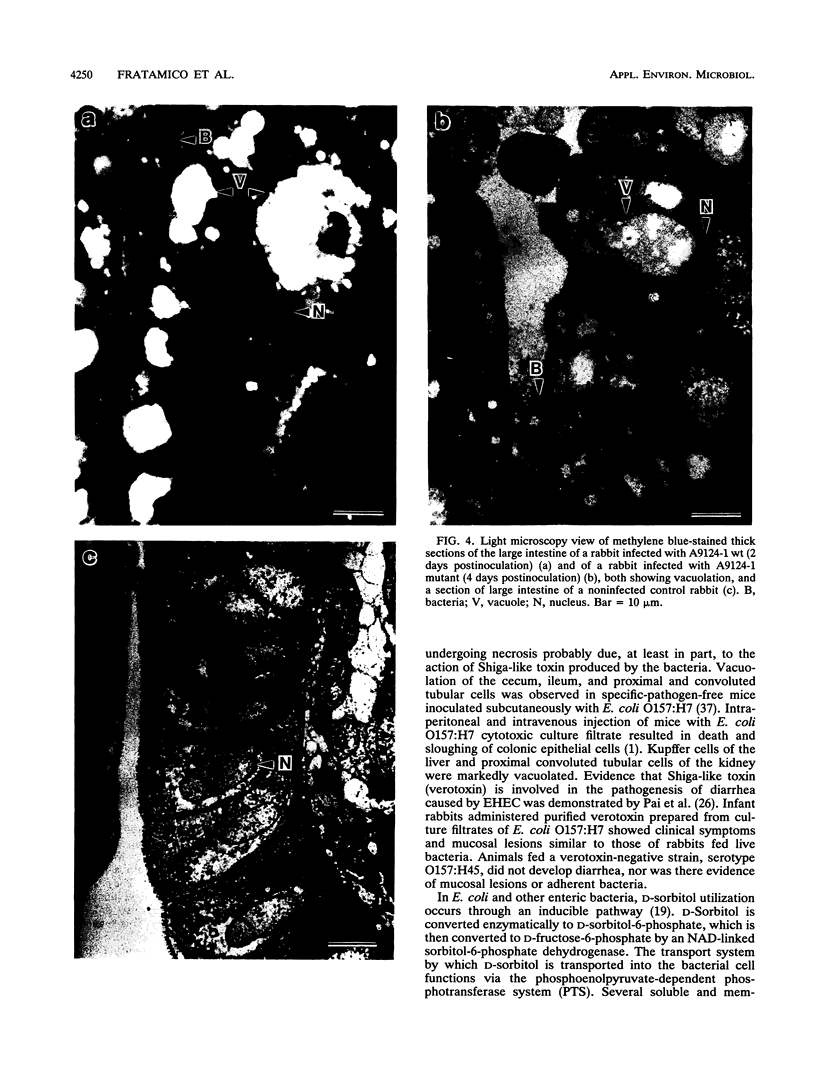
Virulence and pathogenicity of an Escherichia coli O157:H7 sorbitol-positive mutant were investigated with an infant rabbit animal model as well as a battery of in vitro assays. Total cell lysate protein profiles, outer membrane protein profiles, plasmid profiles, and levels of cytotoxic activity against Vero cells were similar in the wild-type and mutant strains. Both adhered to intestinal epithelial cells in culture and reacted with fluorescein isothiocyanate-labeled antiserum against E. coli O157:H7. The mutant appeared to be similar to the wild type in all respects except in its ability to ferment sorbitol. [14C]sorbitol uptake and sorbitol-6-phosphate dehydrogenase activities were notably increased in the mutant strain. Diarrhea developed in rabbits administered the wild-type strain and in those fed the sorbitol-positive mutant. There was greater bacterial attachment and mucosal damage in the cecum and large intestine than in the small intestine. Scanning electron microscopy revealed bacteria adhering as single cells and as aggregates closely associated with mucus. Mucosal lesions consisted of areas of tissue necrosis with sloughing of epithelial cells. By transmission electron microscopy, electron-dense necrotic epithelial cells were visible in areas where bacteria were present, and epithelial cell debris containing bacteria was observed between the villar luminal surfaces. Light microscopy of epithelial cells of intestinal sections of infected rabbits revealed noticeable vacuolation and spherical, pyknotic nuclei. These data indicate that the sorbitol-negative phenotype is not associated with the pathogenicity of E. coli O157:H7.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitzan M., Ludwig K., Klemt M., König H., Büren J., Müller-Wiefel D. E. The role of Escherichia coli O 157 infections in the classical (enteropathic) haemolytic uraemic syndrome: results of a Central European, multicentre study. Epidemiol Infect. 1993 Apr;110(2):183–196. doi: 10.1017/s0950268800068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp C. A., Greene K. D., Downes F. P., Sowers E. G., Wells J. G., Wachsmuth I. K. Unusual verotoxin-producing Escherichia coli associated with hemorrhagic colitis. J Clin Microbiol. 1987 Aug;25(8):1486–1489. doi: 10.1128/jcm.25.8.1486-1489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cordaro J. C., Anderson R. P., Grogan E. W., Jr, Wenzel D. J., Engler M., Roseman S. Promoter-like mutation affecting HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):245–252. doi: 10.1128/jb.120.1.245-252.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Schoeni J. L. Isolation of Escherichia coli O157:H7 from retail fresh meats and poultry. Appl Environ Microbiol. 1987 Oct;53(10):2394–2396. doi: 10.1128/aem.53.10.2394-2396.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Schoeni J. L. Survival and growth characteristics of Escherichia coli associated with hemorrhagic colitis. Appl Environ Microbiol. 1984 Oct;48(4):855–856. doi: 10.1128/aem.48.4.855-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Davis B. R. H7 antiserum-sorbitol fermentation medium: a single tube screening medium for detecting Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1985 Oct;22(4):620–625. doi: 10.1128/jcm.22.4.620-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm R. K., Hinrichs D. J., Thomashow M. F. Introduction of pAM beta 1 into Listeria monocytogenes by conjugation and homology between native L. monocytogenes plasmids. Infect Immun. 1984 Apr;44(1):157–161. doi: 10.1128/iai.44.1.157-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. H., Collins J. E., Duimstra J. R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986 Mar;51(3):953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratamico P. M., Buckley H. R. Identification and characterization of an immunodominant 58-kilodalton antigen of Aspergillus fumigatus recognized by sera of patients with invasive aspergillosis. Infect Immun. 1991 Jan;59(1):309–315. doi: 10.1128/iai.59.1.309-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier F. C., Hayward I., Novotny M. J., Leonard J. E., Saier M. H., Jr Identification of the phosphocarrier protein enzyme IIIgut: essential component of the glucitol phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1985 Mar;161(3):1017–1022. doi: 10.1128/jb.161.3.1017-1022.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzer F., Böhm H., Rüssmann H., Bitzan M., Aleksic S., Karch H. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1992 Jul;30(7):1807–1810. doi: 10.1128/jcm.30.7.1807-1810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman Y., Cohen D., Gluck A., Ohad E., Sechter I. A cluster of cases of Escherichia coli O157 infection in a day-care center in a communal settlement (Kibbutz) in Israel. J Clin Microbiol. 1992 Feb;30(2):520–521. doi: 10.1128/jcm.30.2.520-521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K. L., O'Leary M. J., Cohen M. L., Norris P., Wells J. G., Noll E., Kobayashi J. M., Blake P. A. Escherichia coli O157:H7, an emerging gastrointestinal pathogen. Results of a one-year, prospective, population-based study. JAMA. 1988 Jun 24;259(24):3567–3570. [PubMed] [Google Scholar]
- March S. B., Ratnam S. Latex agglutination test for detection of Escherichia coli serotype O157. J Clin Microbiol. 1989 Jul;27(7):1675–1677. doi: 10.1128/jcm.27.7.1675-1677.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. B., Ratnam S. Sorbitol-MacConkey medium for detection of Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1986 May;23(5):869–872. doi: 10.1128/jcm.23.5.869-872.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- O'Brien A. D., Laveck G. D. Immunochemical and cytotoxic activities of Shigella dysenteriae 1 (shiga) and shiga-like toxins. Infect Immun. 1982 Mar;35(3):1151–1154. doi: 10.1128/iai.35.3.1151-1154.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Kelly J. K., Meyers G. L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986 Jan;51(1):16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. E., Kaufmann A. F., Thomason B. M., Blake P. A., Farmer J. J., 3rd Diarrhea due to Escherichia coli O157:H7 in the infant rabbit. J Infect Dis. 1985 Dec;152(6):1341–1343. doi: 10.1093/infdis/152.6.1341. [DOI] [PubMed] [Google Scholar]
- Ratnam S., March S. B., Ahmed R., Bezanson G. S., Kasatiya S. Characterization of Escherichia coli serotype O157:H7. J Clin Microbiol. 1988 Oct;26(10):2006–2012. doi: 10.1128/jcm.26.10.2006-2012.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Newland J. W., Smith H. W., Holmes R. K., O'Brien A. D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986 Jul;53(1):135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow D. L., Woodruff B. A., Brady R. C., Griffin P. M., Tippen S., Donnell H. D., Jr, Geldreich E., Payne B. J., Meyer A., Jr, Wells J. G. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann Intern Med. 1992 Nov 15;117(10):812–819. doi: 10.7326/0003-4819-117-10-812. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Karch H., Wachsmuth K. I., Robins-Browne R. M., O'Brien A. D., Lior H., Cohen M. L., Smithers J., Levine M. M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987 Dec;55(12):3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. G., Davis B. R., Wachsmuth I. K., Riley L. W., Remis R. S., Sokolow R., Morris G. K. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J Clin Microbiol. 1983 Sep;18(3):512–520. doi: 10.1128/jcm.18.3.512-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor D. K., Jr, Ashkenazi S., Chiovetti R., Cleary T. G. Adherence of enterohemorrhagic Escherichia coli strains to a human colonic epithelial cell line (T84). Infect Immun. 1992 Apr;60(4):1613–1617. doi: 10.1128/iai.60.4.1613-1617.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]