Abstract
Depression is a frequent comorbidity in epilepsy patients. A variety of biological factors may underlie epilepsy-associated depression. We examined whether kindling-induced chronic increase in seizure susceptibility is accompanied by behavioral symptoms of depression. Three week-old Wistar rats underwent rapid kindling - 84 initially subconvulsant electrical stimulations of ventral hippocampus delivered every five minutes - followed by depression-specific behavioral tests performed two and four weeks later. Kindled animals exhibited sustained increase in the immobility time in the forced swim test and the loss of taste preference towards calorie-free saccharin, as compared to controls. Initial loss of preference towards the intake of calorie-containing sucrose was followed by the increased consumption at four weeks. At both time points, animals exhibited enhanced seizure susceptibility upon test stimulations of the hippocampus. We conclude that neuronal plastic changes associated with kindling state are accompanied by the development of depressive behavior.
Keywords: Epilepsy, depression, kindling, forced swim test, taste preference, rat
1. Introduction
Depression has been identified as the most frequent psychiatric comorbidity in patients with epilepsy and the possibility of a neurobiological connection between epilepsy and depression has entered recent discussions on this topic [1–3]. The prevalence of lifetime-to-date major depression among patients with epilepsy ranged from 8 to 48 per cent [4]. A history of depression was seven times more frequent in patients newly diagnosed with epilepsy than among age- and sex-matched controls in a population-based case-control study [5]. In a recent study of 171 children with epilepsy, Caplan and coworkers [6] found a prevalence rate of affective and anxiety disorders to be 33% compared to 6% in the control group of children without epilepsy. In that study, more than twice as many children with epilepsy (20% compared to 9% of control) reported suicidal ideation. Interictal psychiatric symptoms adversely influence the health-related quality of life to a greater extent than the frequency, severity and chronicity of seizures [7]. Consideration of the psychiatric comorbidities in treatment decisions involving drug selection has been discussed for both adult [8] and pediatric [9] patients with epilepsy.
The relationship between mood disorders and types of epilepsy has been addressed in several studies. Piazzini et al. [10] reported a greater prevalence of depression and anxiety in those with partial epilepsy than those with generalized epilepsy (temporal lobe epilepsy > frontal lobe epilepsy > idiopathic generalized epilepsy > controls). On the other hand, Ott et al. [11] found that children with complex partial seizures, including TLE and idiopathic generalized epilepsy of the absence type have had similar rates of depression. TLE and depression appear to share several mechanisms, including impairments in serotonergic, noradrenergic, glutamatergic and GABAergic transmission [12–15].
Despite the apparent connections, the mechanisms of depression in epilepsy patients are poorly understood. It is not known whether depression in epilepsy patients is adaptational (reactive) to the psychosocial aspects of being in the epileptic state, or whether it has a neurobiological basis. Furthermore studies of depression in epilepsy patients are confounded by multiple factors, including antiepileptic drug therapy, psychosocial, familial, socioeconomic, and intellectual effects. Human studies are complicated by many factors that limit our ability to understand the relative contribution of the neurobiological relationship between depression and epilepsy.
Investigating depression in animal models of chronic epilepsy is also complicated. Epileptogenesis in animals after experimental status epilepticus is often accompanied by significant brain damage such that it is not clear whether the anatomic lesion itself may contribute to behavioral alterations. Behavioral testing can be affected by recurring seizures. It is important to establish, whether epileptic state (increased seizure susceptibility) per se, rather than epileptic seizures and morphological changes is accompanied by symptoms of depression.
Kindling is a model of limbic epilepsy, which is characterized by sustained increase in seizure susceptibility. Kindling is different from post-status epilepticus TLE in two major respects: the absence or minimal extent of neuronal injury; the absence of spontaneous recurrent seizures. At the same time, kindled animals exhibit persistent enhanced seizure susceptibility. Hence, kindling appears to be especially suitable for studying epilepsy-associated depression that results from neuronal plastic changes. A limitation of traditional kindling protocols is that rapid brain and skull growth in developing animals displace electrodes. Lothman and co-workers [16, 17] described rapid kindling, which affords creating the state of enhanced susceptibility within several hours. We have adapted this approach to evaluating age-specific and pharmacologic properties of epileptogenesis [18, 19].
No studies of kindling epileptogenesis and behavioral correlates of depression have been performed in immature animals. The goal of the present study was to examine whether enhanced seizure susceptibility produced by rapid kindling in immature rats in the absence of neuronal injury and spontaneous seizures is accompanied by behavioral symptoms of depression.
2. Methods
2.1. Animals
The experiments were performed on male Wistar rats (Charles River, Wilmington, MA), twenty days of age at the beginning of the study. The experiments were done in accordance with the policies of the National Institutes of Health and the UCLA Office for the Protection of Research Subjects.
The experimental design is outlined in Figure 1. The study included the following experimental groups. 1: Kindling followed by afterdischarge and seizure examination 2 weeks later (n=5); these animals were not used in behavioral trials, to avoid possible effects of repeated surgery and test stimulation on behavior; 2: Kindling followed by behavioral tests 2 and 4 weeks later, with afterdischarge and seizure examination after the second behavioral trial (n=9); 3: Sham (n=5); these animals underwent surgery, but were not subjected to kindling, and were used only for behavioral tests; 4: Naïve rats (n=6) subjected to behavioral trials only.
Figure 1. Experimental design.
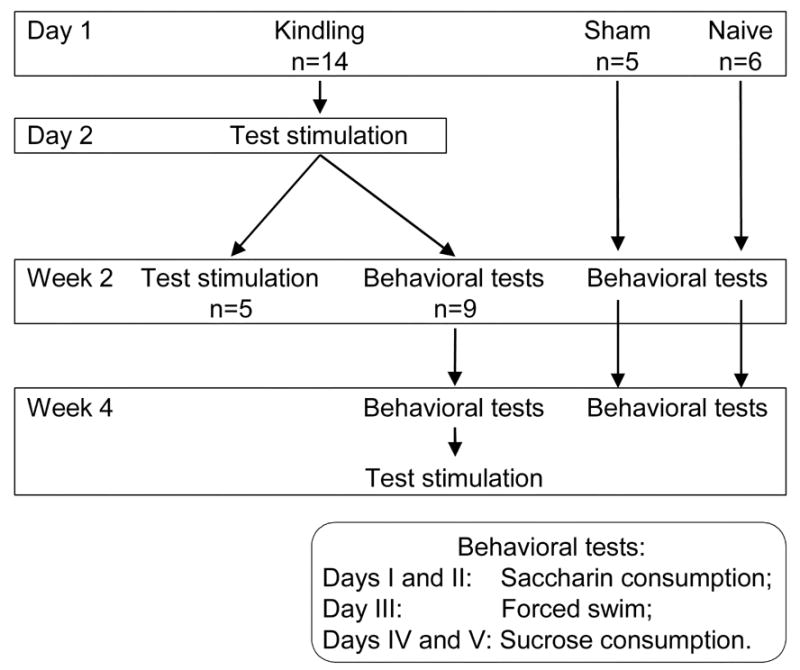
At postnatal day 21, rats were subjected to rapid kindling followed 24 hours later by test stimulations to measure afterdischarge properties and seizure response. Two weeks after kindling, afterdischarge properties and seizure response to test stimulation were examined in a subset of these animals (n=5). The remaining nine animals underwent behavioral tests two and four weeks after kindling; in these rats, response to test stimulation was examined four days after the completion of behavioral studies. In addition, behavior was examined in five sham and six naïve animals. The sequence of tests during each behavioral trial is indicated in the bottom inset.
2.2. Surgery
Under Isoflurane anesthesia, the animals were stereotaxically implanted with a bipolar stimulating electrode (Plastics1 Inc., Roanoke, VA) in the left ventral hippocampus (2.9 mm posterior and 3.7 mm left from Bregma; 3.8 mm ventral from the brain surface [18, 20]). A tripolar recording electrode (Plastics1 Inc.) was wrapped around skull screws using the nasal bone as the ground. Electrodes were fixed to the skull with Cerebond adhesive (MyNeurolab.com, St. Louis, MO). After surgery, animals were weaned and housed individually in cages, and were given food and water ad libitum in a temperature controlled room (24–26°C) with a twelve hour light-dark cycle.
2.3. Kindling and testing of seizure susceptibility in kindled rats
Twenty four hours after surgery, the animals were subjected to the rapid kindling procedure [18, 20] . The animals were connected to the DS8000 electrical stimulator via DS100 stimulus isolators (World Precision Instruments Inc., Sarasota, FL) and to the MP100/EEG100B acquisition system (BIOPAC Systems Inc., Santa Barbara, CA). EEG was acquired using AcqKnowledge 3.82 software (BIOPAC) along with simultaneous digital video. Both EEG and behavioral responses were analyzed off-line. Throughout the procedure, animals were kept individually in Plexiglas observation chambers, equipped with water bottles and feeders (Instech Laboratories, Plymouth Meeting, PA).
At the beginning of the experiment, afterdischarge threshold and duration were detected by applying electrical stimuli consisting of 10 s train duration, 20 Hz, one ms pulse duration, square wave monophasic stimuli, starting at 0.2 mA, at 0.1 mA increments, delivered every 10 minutes. The rapid kindling procedure, which started 10 minutes after detecting the afterdischarge, consisted of 84 trains delivered every five minutes using the parameters described above, at a current of 0.1 mA over the afterdischarge threshold; the total procedure duration was 7 hours. Behavioral seizures were scored using the following scale: 1- Motor arrest and twitching vibrissae; 2 – chewing, head bobbing; 3- forelimb clonus; 4- forelimb clonus and rearing; 5- rearing and falling.
Kindled rats were then retested at twenty four hours for their afterdischarge properties and seizure response to threshold stimulation. The electrode caps were subsequently removed, and wounds were closed with nonabsorbable, monofilament surgical sutures. Two weeks after the initial procedure, some kindled rats (n=5) were retested for their afterdischarge properties and seizure response to threshold stimulation by reimplanting them with a recording electrode as described earlier, and a stimulating electrode with placement adjusted to account for the growth of the brain/change of skull shape (3.6 mm posterior, 4.9 mm left from Bregma, 5.0 mm ventral from brain surface [18]. The remaining rats that had been kindled earlier (n=9), were used for behavioral testing described below. However, these rats were not reimplanted until after the behavioral tests in order to avoid the effects of surgery or the test stimulation itself, on their behavioral responses.
2.4. Behavioral tests
Forced swim test (FST) is one of the most widely used protocols for studying both pharmacological and pathophysiological aspects of depression [21–25]. FST creates a situation of despair, and allows evaluation of the ability of the animal to adopt active strategies in inescapable stressful situation; failure to do so is indicative of a depression-like state [21, 25] . We employed a modified FST, when the animals underwent a single five-minute trial [26]; such a modification of the classic Porsolt test [22] was shown to be relevant for both examining depressive state, and for screening antidepressant agents. Furthermore, since the first swim trial under conditions of classic Porsolt test plays a role of depressogenic treatment [22], such a procedure might complicate the interpretation of behavioral data in the already depressed animals.
The test was performed between 9:00AM and 1:00PM. In each trial, the animal was placed for 5 minutes in a plastic bucket (40 cm in height and 30 cm in width) filled with tap water maintained at 25°C, to a height of 30 cm. After each test, the container was washed and refilled with fresh water. The swim behavior was videotaped and analyzed offline for the total immobility time by an investigator blinded to treatment. Immobility was defined as moving the limbs only enough to stay above water, as opposed to escaping or exploring behavior (trying to escape from the tank) or exploring behavior (swimming along the wall, across the tank, or diving). After the test, the rats were dried and returned to their cages.
One of core symptoms of depression is the loss of the ability to experience pleasure, referred to as anhedonia [21, 26, 27]. In rats, anhedonia can be assessed by the loss of taste preference; while normal animals prefer sweetened water over regular water, animals with depression do not exhibit such a preference [23, 24, 28–30].
Taste preference was examined using both saccharin (calorie-free) and sucrose (calorie-containing) consumption tests [23, 29, 31, 32]. The animals had free access to a standard rodent diet. Each cage was supplied with two identical graduated water bottles, each containing 250 ml of water. On the day of the test, regular water in one of the bottles was replaced with either 0.1% saccharin, or 1% sucrose (Sigma, St. Louis, MO) diluted in tap water. A bottle of normal drinking water plus a bottle of either saccharin- or sucrose- containing bottle were coded and randomly placed in the cages by a “blinded” investigator. Saccharin- or sucrose-containing bottles were positioned in different slots to avoid habituation.
The test was performed starting from 5:00PM and ran for 24 hours, after which saccharin/sucrose solution was replaced with normal drinking water.
Both behavioral tests were repeated two weeks later, after which previously kindled rats were reimplanted with stimulating/recording electrodes to test their long-term afterdischarge and seizure behavior responses. The total volume of consumed fluid and percent of consumed saccharin or sucrose solution of the total consumed fluid were calculated. Both sham and naïve rats served as concurrent controls for both behavioral tests.
2.5. Data analysis
Data were analyzed using Prizm 4 software (GraphPad, San Diego, CA). The choice of tests was based on the scales (ordinal or interval) and the normality of distribution. The tests employed for data analysis and comparison are indicated in figure legends.
3. Results
3.1. Kindling
The first kindling stimulation induced no behavioral seizures in any of animals. Repetitive electrical stimulations led to the occurrence and progressive development of seizure responses, so that by the end of the procedure, animals exhibited 36±1.4 stage 4–5 convulsions (minimum 30, maximum 45; data are combined for all 14 kindled rats). Twenty four hours after the end of kindling, all animals exhibited a statistically significant reduction of the afterdischarge threshold, and an increase of afterdischarge duration, as compared to baseline values (Fig. 2 A, B). Furthermore, eight animals developed stage 4 seizures, and the remaining six animals exhibited stage 3 convulsions (Fig. 2C). For further, studies animals were divided in two groups in such a way, that the distribution of animals with stage 3 and stage 4 seizures was similar between the groups used for test stimulation and behavioral studies (Fig. 1; test stimulation- two rats with stage 3 and three rats with stage 4; behavioral tests- 4 animals with stage 3 and five animals with stage 4).
Figure 2. Afterdischarge threshold (A), duration (B) and seizure responses (C) in the animals before and after rapid kindling.
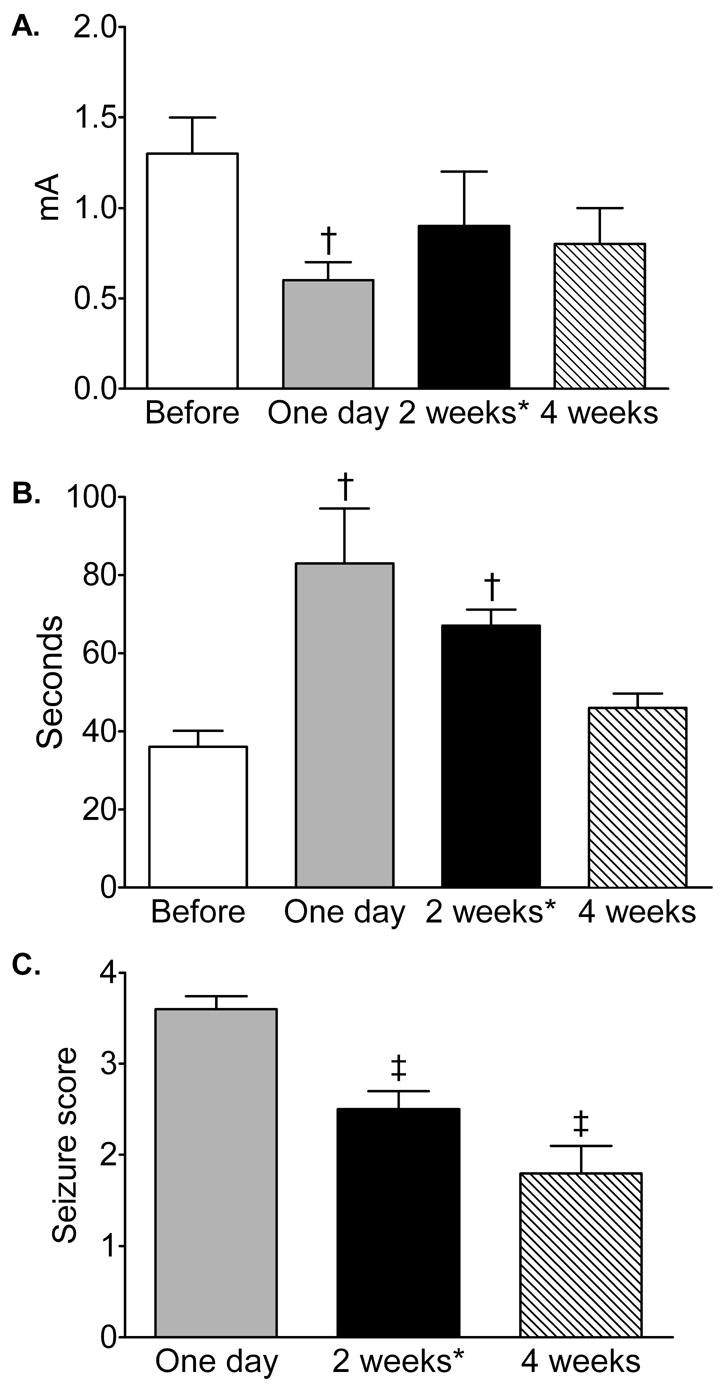
Data before and one day after kindling are pooled for 14 animals for presentation purposes. Afterdischarge and seizure parameters two weeks (n=5) and four weeks (n=9) after kindling are compared for respective animals. Animals were divided for 2 and 4 weeks tests in such a way, that there were no differences in the examined parameters between the two subgroups. X-axis refers to time points with the reference to kindling. *-these animals were not used for behavioral tests. Data are presented as Mean±SEM. †- p<0.05 vs. “Before kindling” (Friedman+Wilcoxon test). ‡- p<0.05 vs. One day after kindling (Wilcoxon test).
Two weeks after kindling the afterdischarge threshold returned to the baseline value, while afterdischarge duration remained increased; the animals developed stage 2–3 seizures in response to threshold stimulation. Four weeks after kindling both afterdischarge parameters were not different from baseline; however 8 out of nine animals developed stage 1–3 seizures in response to the threshold stimulation (Fig. 2A-C).
3.2. Forced swim test
Both naïve and sham animals showed similar immobility time during both two- and four-week recording sessions (Fig. 3A, p>0.05). When the data between the two groups were combined, minimal immobility time was 75 s and maximal was 140 s with a median 100 s. At the same time, kindled animals spent significantly more time immobile in the tank at both two weeks after (Fig. 3A, p<0.05 vs. both naïve and sham, minimum 145 s, maximum 230 s, median 185 s), and four weeks after kindling (minimum 140 s, maximum 220 s, median 165 s, Fig. 3A), as compared to controls. There were no differences in immobility time in the kindled animals between the tests performed at 2 and 4 weeks.
Figure 3. Forced swim test in the animals before and after rapid kindling.
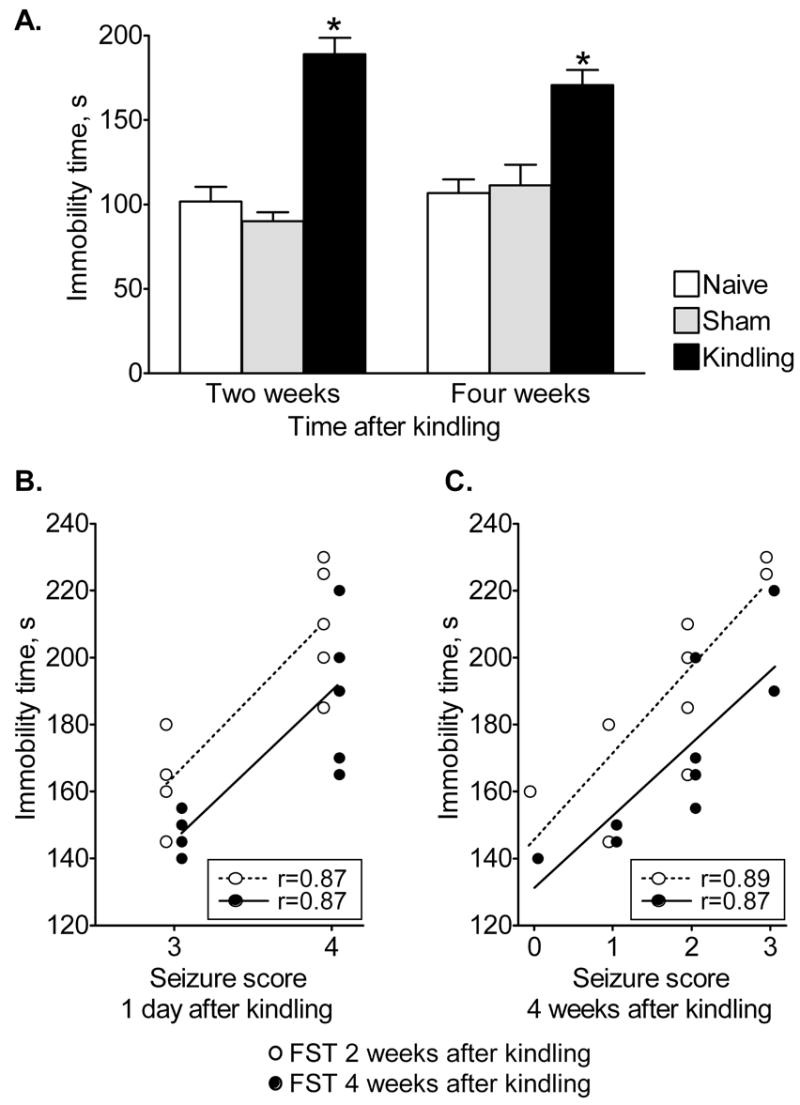
A. Mean±SEM values two and four weeks after kindling. *-p<0.05 vs. both naïve and sham (One-way ANOVA+Bonferroni test). There were no statistical differences (p>0.1) between two and four weeks for any of groups (paired t-test). Seizure score in individual animals in response to threshold stimulation one day (B) and four weeks (C) after kindling is plotted against immobility time in FST 2 weeks (open circles) and 4 weeks (black circles) after kindling. Coefficient of correlation (r) was calculated using Spearman test, is indicated for each of the data set; positive correlation was statistically significant in all cases (p<0.05).
We further examined whether the immobility time under conditions of FST correlated with the severity of behavioral seizures in response to test stimulations in individual animals. We found a strong positive correlation (p<0.05) between the time spent immobile during FST both 2 and 4 weeks after kindling, and seizure score both 24 hours and 4 weeks after kindling (Fig. 3).
3.3. Total fluid, saccharin and sucrose consumption
There were no statistically significant differences in the daily consumption of water among the three groups at each of the examined ages, although the total volume of consumed water increased with age (naive- 60±3.5 ml at five weeks and 74±2.4 ml at seven weeks of age; sham- 55±2.9 ml two weeks, and 71±3.1 four weeks after sham procedure; kindling- 55±3.2 two weeks and 72±2.3 ml four weeks after kindling).
Introduction of saccharin did not lead to the increase of total volume of consumed fluid in any of experimental groups (Fig. 4A). However, both naïve and sham rats exhibited strong preference for saccharin solution over tap water during both two- and four-week tests. The kindled animals did not exhibit a preference for saccharin solution in the two- and four-week tests (Fig. 4B).
Figure 4. Saccharin (A, B) and sucrose (C, D) consumption over twenty-four hour period two and four weeks after rapid kindling.
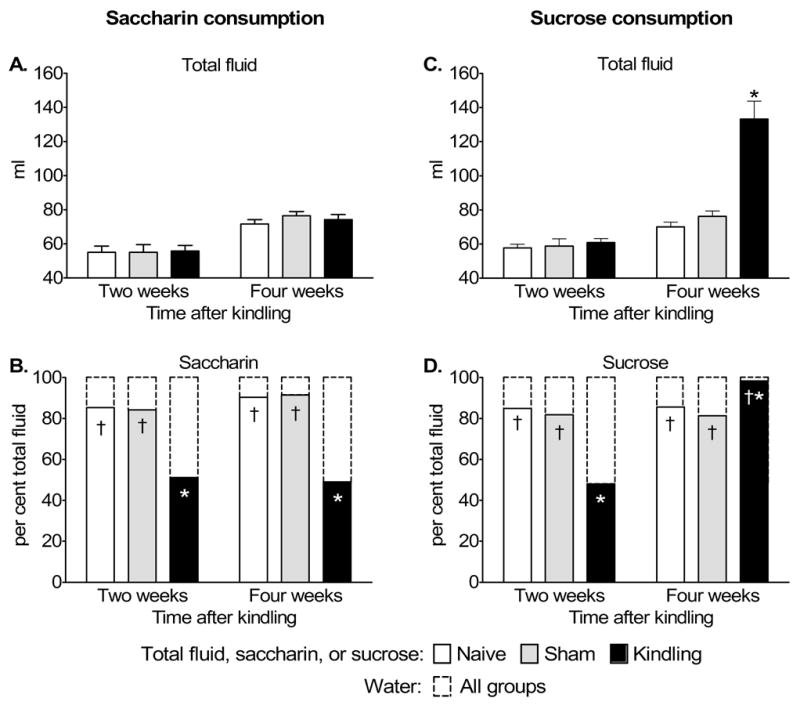
A. No differences were observed at any age in total fluid (tap water +saccharin) intake among control and experimental groups. B. While naïve and sham animals exhibited strong preference towards saccharin versus tap water, kindled animals exhibited loss of taste preference towards saccharin both 2 and 4 weeks after kindling. C. No differences were observed in total fluid (tap water + sucrose) intake between naïve and sham animals. However, the total volume of consumed fluid was significantly larger in kindled animals. D. Both naïve and sham animals preferred sucrose over tap water. Kindled animals did not show sucrose preference 2 weeks after kindling. However, at four weeks, kindled animals consumed significantly more of sucrose solution as compared to both sham and naïve rats. Data in A and C are presented as Mean±SEM, and on B and D- as Mean. *-p<0.05 vs. both Naïve and sham (One way ANOVA + Bonferroni); †- p<0.05 of per cent of either saccharin or sucrose (bottom bars in the stacks) versus percent of water (top bars in the stacks, T test).
In naïve and sham animals, introduction of sucrose solution, did not lead to the increase of the total daily volume of consumed fluid (Fig. 4C). Similar to the experiments involving saccharin, animals of both naïve and sham groups exhibited a strong preference towards sucrose solution at both examination time points (Fig. 4D). In contrast to either of the control groups, kindled animals did not exhibit any preference towards sucrose solution two weeks after stimulation, while the total volume of consumed fluid was not different from controls (Fig. 4C, D). However, four weeks after kindling, these animals consumed significantly more fluid than either naïve or sham rats, almost entirely consisting of the sucrose solution (98±1.7% of the total fluid consumed over 24 hours) (Fig. 4C,D).
No statistically significant correlation was revealed between the seizure responses both 1 day and four weeks after kindling and the percent of saccharin and sucrose consumption.
4. Discussion
Our data provide evidence that rapid kindling in young animals is accompanied by sustained changes in forced swimming and taste preference tests that can be interpreted as behavioral correlates of depression.
In contrast to conventional kindling, rapid kindling is not regarded as a permanent condition: while conventional kindling leads to enhanced brain excitability and the susceptibility to seizures lasts for the lifetime [33], the changes after rapid kindling tend to disappear gradually [17]. Indeed, in our studies, afterdischarge threshold returned to baseline values in as early as two weeks, and afterdischarge duration- four weeks after kindling. Yet, certain key long-lasting features of kindling state were observed after rapid kindling. Increased afterdischarge duration was recorded two weeks after kindling. Threshold stimulation, which had been subconvulsant in naïve animals, evoked behavioral seizure responses both at 2 and 4 weeks after kindling (even though the severity of seizures declined as a function of time). Thus, rapid kindling is capable of inducing neural plasticity that lasts for at least several weeks, and which translates into the enhanced seizure response.
An important advantage of rapid kindling over conventional protocol is the “compressed” duration of epileptogenesis, which allows reproducing the progression of epileptic process within certain developmental stage of the animal. This is not possible with conventional kindling, since rapid ontogenic development of experimental animals outpaces the evolution of kindling process. In contrast to post-status epilepticus epilepsy, kindling is not accompanied by hippocampal sclerosis and spontaneous seizures. Eliminating spontaneous seizures as a variable permits this model to accommodate an important clinical feature of epilepsy-associated depression observed by Attarian et al [34] and Quigg et al [35]. Those authors found that the prevalence depression was independent of the degree of seizure control. Caplan and associates [6] did not find an association between seizure variables and affective disorders in children. Rapid kindling provides an opportunity to focus on neuronal plastic changes that underlie behavioral abnormalities without concerns regarding if behavioral testing was affected by post-ictal state et cetera.
Reports on depression-like disorders associated with experimental limbic epilepsy (an experimental correlate of TLE), particularly kindling, are scarce and controversial. Cannizzaro et al [36], Sabatino et al [37], Helfer et al [38], Ma and Leung [39], Witnick et al [40], did not find changes in FST and in taste preference in the animals kindled with either pentylenetetrazole, or amygdala stimulation. In contrast, Mortazavi et al [41] found that pentylenetetrazole-kindled animals showed increased immobility time in FST. Animals in that study received chronic chemical provocation with pentylenetetrazole and had sustained hippocampal injury.
Correlating symptoms of depression in humans with behavioral patterns of depression in the rat is a challenge, and cannot rely on a single behavioral test. Similar to humans, in whom depression is associated with behavioral, vegetative, and biochemical variables [27], experimental animals should exhibit a variety of behavioral, vegetative, and biochemical abnormalities in order to satisfy the definition of depression; the importance of a combination approach in evaluating depression in animal models is emphasized by the fact that an important symptom of depression, suicidality, cannot be reproduced in experimental animals. We used two behavioral tests, which are most commonly applied for studying depressive behavior in rodents: FST and taste preference.
Rapid kindling led to the sustained changes in FST. Kindled animals showed significant increase in immobility time. Interestingly, the extent of these changes positively correlated with the severity of behavioral seizures elicited by threshold stimulation on both one day and 4 weeks after kindling in individual animals. This suggests that even though animals did not exhibit spontaneous seizures, the strength of neuronal plastic changes (that translated to the severity of seizures in response to threshold stimulation) affects the extent of such feature of depression as the inability to adopt an active strategy under an inescapable stressful situation.
Loss of taste preference which can be interpreted as anhedonia was also observed in kindled animals. In saccharin consumption test, this was observed at both 2 and 4 weeks after rapid kindling. Surprisingly, in the sucrose consumption test 4 weeks after kindling, the animals’ behavior was reversed from the loss of preference to enhanced preference, even if compared to naïve subjects. In contrast to saccharin, sucrose has caloric value. It has been known that depression can be accompanied by both loss and the increase of appetite [21, 27]; furthermore, antidepressant drugs, such as selective serotonin reuptake inhibitors, are commonly known to reduce appetite and curb weight gain [42–44]. Since the increase of taste preference in sucrose test 4 weeks after kindling can be excluded based on saccharin test, it is conceivable that increased sucrose intake reflects increased appetite. Studies are underway with food consumption to confirm this suggestion.
In summary, the important findings in this report are: 1) the evolution of depressive behavior accompanying epileptogenesis can be modeled in young animals 2) epilepsy associated depressive behavior can be demonstrated in this model without either overt brain injury or ongoing spontaneous seizures. Upon further validation, which would include both expanded behavioral and biochemical assays, our model can provide a good platform for evaluating pharmacological interventions to treat depression in epilepsy.
Acknowledgments
Supported by NIH grants NS043409 (AM), NS046516 (RS), NS32070 and MH067187 (RC), the DAPA Foundation (RS), and Association pour l'Etude des Affections Congenitales (AEAC) (SA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilliam FG, Santos J, Vahle V, Carter J, Brown K, Hecimovic H. Depression in epilepsy: ignoring clinical expression of neuronal network dysfunction? Epilepsia. 2004;45(Suppl 2):28–33. doi: 10.1111/j.0013-9580.2004.452005.x. [DOI] [PubMed] [Google Scholar]
- 2.Kanner AM. Depression in epilepsy: a neurobiologic perspective. Epilepsy Curr. 2005;5:21–27. doi: 10.1111/j.1535-7597.2005.05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanner AM. Epilepsy, suicidal behaviour, and depression: do they share common pathogenic mechanisms? Lancet Neurol. 2006;5:107–8. doi: 10.1016/S1474-4422(06)70331-3. [DOI] [PubMed] [Google Scholar]
- 4.Hermann BP, Seidenberg M, Bell B. Psychiatric comorbidity in chronic epilepsy: identification, consequences, and treatment of major depression. Epilepsia. 2000;41 (Suppl 2):31–41. doi: 10.1111/j.1528-1157.2000.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 5.Forsgren L, Nystrom L. An incident case-referent study of epileptic seizures in adults. Epilepsy Res. 1990;6:66–81. doi: 10.1016/0920-1211(90)90010-s. [DOI] [PubMed] [Google Scholar]
- 6.Caplan R, Siddarth P, Gurbani S, Hanson R, Sankar R, Shields WD. Depression and anxiety disorders in pediatric epilepsy. Epilepsia. 2005;46:720–30. doi: 10.1111/j.1528-1167.2005.43604.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia. 2004;45:544–50. doi: 10.1111/j.0013-9580.2004.47003.x. [DOI] [PubMed] [Google Scholar]
- 8.Harden CL. The co-morbidity of depression and epilepsy: epidemiology, etiology, and treatment. Neurology. 2002;59(Suppl 4):48–55. doi: 10.1212/wnl.59.6_suppl_4.s48. [DOI] [PubMed] [Google Scholar]
- 9.Sankar R. Initial treatment of epilepsy with antiepileptic drugs: pediatric issues. Neurology. 2004;63 (Suppl 4):S30–S39. doi: 10.1212/wnl.63.10_suppl_4.s30. [DOI] [PubMed] [Google Scholar]
- 10.Piazzini A, Canevini MP, Maggiori G, Canger R. Depression and Anxiety in Patients with Epilepsy. Epilepsy Behav. 2001;2:481–89. doi: 10.1006/ebeh.2001.0247. [DOI] [PubMed] [Google Scholar]
- 11.Ott D, Caplan R, Guthrie D, Siddarth P, Komo S, Shields WD, et al. Measures of psychopathology in children with complex partial seizures and primary generalized epilepsy with absence. J Am Acad Child Adolesc Psychiatry. 2001;40:907–14. doi: 10.1097/00004583-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–92. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 13.Savic I, Lindstrom P, Gulyas B, Halldin C, Andree B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62:1343–51. doi: 10.1212/01.wnl.0000123696.98166.af. [DOI] [PubMed] [Google Scholar]
- 14.Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, Der MG, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–56. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- 15.Jobe PC. Common pathogenic mechanisms between depression and epilepsy: an experimental perspective. Epilepsy Behav. 2003;4:S14–S24. doi: 10.1016/j.yebeh.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Lothman EW, Hatlelid JM, Zorumski CF, Conry JA, Moon PFJBP. Kindling with rapidly recurring hippocampal seizures. Brain Res. 1985;360:83–91. doi: 10.1016/0006-8993(85)91223-5. [DOI] [PubMed] [Google Scholar]
- 17.Lothman EW, Williamson JM. Closely spaced recurrent hippocampal seizures elicit two types of heightened epileptogenesis: a rapidly developing, transient kindling and a slowly developing, enduring kindling. Brain Res. 1994;649:71–84. doi: 10.1016/0006-8993(94)91050-2. [DOI] [PubMed] [Google Scholar]
- 18.Mazarati AM, Shin DH, Auvin S, Sankar R. Age-dependent effects of topiramate on the acquisition and the retention of rapid kindling. Epilepsia. 2007;48 doi: 10.1111/j.1528-1167.2007.00987.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazarati AM, Sollenberg U, Lundström L, Shin D, Langel U, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: the effects of subtype selective agonists and the role of G-protein mediated signaling. J Pharmacol Exp Ther. 2006;318:700–08. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- 20.Michelson HB, Lothman EW. An ontogenetic study of kindling using rapidly recurring hippocampal seizures. Dev Brain Res. 1991;61:79–85. doi: 10.1016/0165-3806(91)90116-z. [DOI] [PubMed] [Google Scholar]
- 21.Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–59. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol. 1979;57:201–10. doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- 23.Sarkisova KY, Midzianovskaia IS, Kulikov MA. Depressive-like behavioral alterations and c-fos expression in the dopaminergic brain regions in WAG/Rij rats with genetic absence epilepsy. Behav Brain Res. 2003;144:211–26. doi: 10.1016/s0166-4328(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 24.Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13:169–88. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cognit Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- 26.Zangen A, Nakash R, Overstreet DH, Yadid G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 2001;155:434–9. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- 27.American Psychaitric Association. Diagnostic and statistical manual of mental disorders. 4. 2000. Mood Disorders; pp. 345–429. [Google Scholar]
- 28.Moreau JL. Validation of an animal model of anhedonia, a major symptom of depression. Encephale. 1997;23:280–89. [PubMed] [Google Scholar]
- 29.Malkesman O, Braw Y, Zagoory-Sharon O, Golan O, Lavi-Avnon Y, Schroeder M, et al. Reward and anxiety in genetic animal models of childhood depression. Behav Brain Res. 2005;164:1–10. doi: 10.1016/j.bbr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav. 1993;54:1215–20. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- 31.Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, et al. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–77. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Gronli J, Murison R, Bjorvatn B, Sorensen E, Portas C, Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behav Brain Res. 2004;150:139–47. doi: 10.1016/S0166-4328(03)00252-3. [DOI] [PubMed] [Google Scholar]
- 33.Stafstrom CE, Sutula TP. Models of epilepsy in the developing and adult brain: implications for neuroprotection. Epilepsy Behav. 2005;7(Suppl 3):S18–S24. doi: 10.1016/j.yebeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Attarian H, Vahle V, Carter J, Hykes E, Gilliam F. Relationship between depression and intractability of seizures. Epilepsy Behav. 2003;4:298–301. doi: 10.1016/s1525-5050(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 35.Quigg M, Broshek DK, Heidal-Schiltz S, Maedgen JW, Bertram EH., 3rd Depression in intractable partial epilepsy varies by laterality of focus and surgery. Epilepsia. 2003;44:419–24. doi: 10.1046/j.1528-1157.2003.18802.x. [DOI] [PubMed] [Google Scholar]
- 36.Cannizzaro G, Flugy A, Cannizzaro C, Gagliano M, Sabatino M. Effects of desipramine and alprazolam in the forced swim test in rats after long-lasting termination of chronic exposure to picrotoxin and pentylenetetrazol. Eur Neuropsychopharmacol. 1993;3:477–84. doi: 10.1016/0924-977x(93)90272-n. [DOI] [PubMed] [Google Scholar]
- 37.Sabatino M, Cannizzaro C, Flugy A, Gagliand M, Mineo A, Cannizzaro G. NMDA-GABA interactions in an animal model of behaviour: a gating mechanism from motivation toward psychotic-like symptoms. Eur Neuropsychopharmacol. 1994;4:103–9. doi: 10.1016/0924-977x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 38.Helfer V, Deransart C, Marescaux C, Depaulis A. Amygdala kindling in the rat: anxiogenic-like consequences. Neuroscience. 1996;73:971–8. doi: 10.1016/0306-4522(96)00081-4. [DOI] [PubMed] [Google Scholar]
- 39.Ma J, Leung LS. Schizophrenia-like behavioral changes after partial hippocampal kindling. Brain Res. 2004;997:111–18. doi: 10.1016/j.brainres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Wintink AJ, Young NA, Davis ACAG, Kalynchuk LE. Kindling-induced emotional behavior in male and female rats. Behav Neurosci. 2003;117:632–40. doi: 10.1037/0735-7044.117.3.632. [DOI] [PubMed] [Google Scholar]
- 41.Mortazavi F, Ericson M, Story D, Hulce VD, Dunbar GL. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005;7:629–38. doi: 10.1016/j.yebeh.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Grignaschi G, Samanin R. Role of serotonin and catecholamines in brain in the feeding suppressant effect of fluoxetine. Neuropharmacology. 1992;31:445–49. doi: 10.1016/0028-3908(92)90082-z. [DOI] [PubMed] [Google Scholar]
- 43.Heisler LK, Kanarek RB, Gerstein A. Fluoxetine decreases fat and protein intakes but not carbohydrate intake in male rats. Pharmacol Biochem Behav. 1997;58:767–73. doi: 10.1016/s0091-3057(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 44.Luo SQ, Li ET. Effects of repeated administration of serotonergic agonists on diet selection and body weight in rats. Pharmacol Biochem Behav. 1991;38:495–500. doi: 10.1016/0091-3057(91)90003-k. [DOI] [PubMed] [Google Scholar]


