Abstract
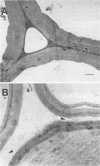
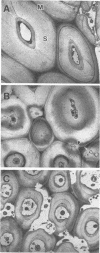
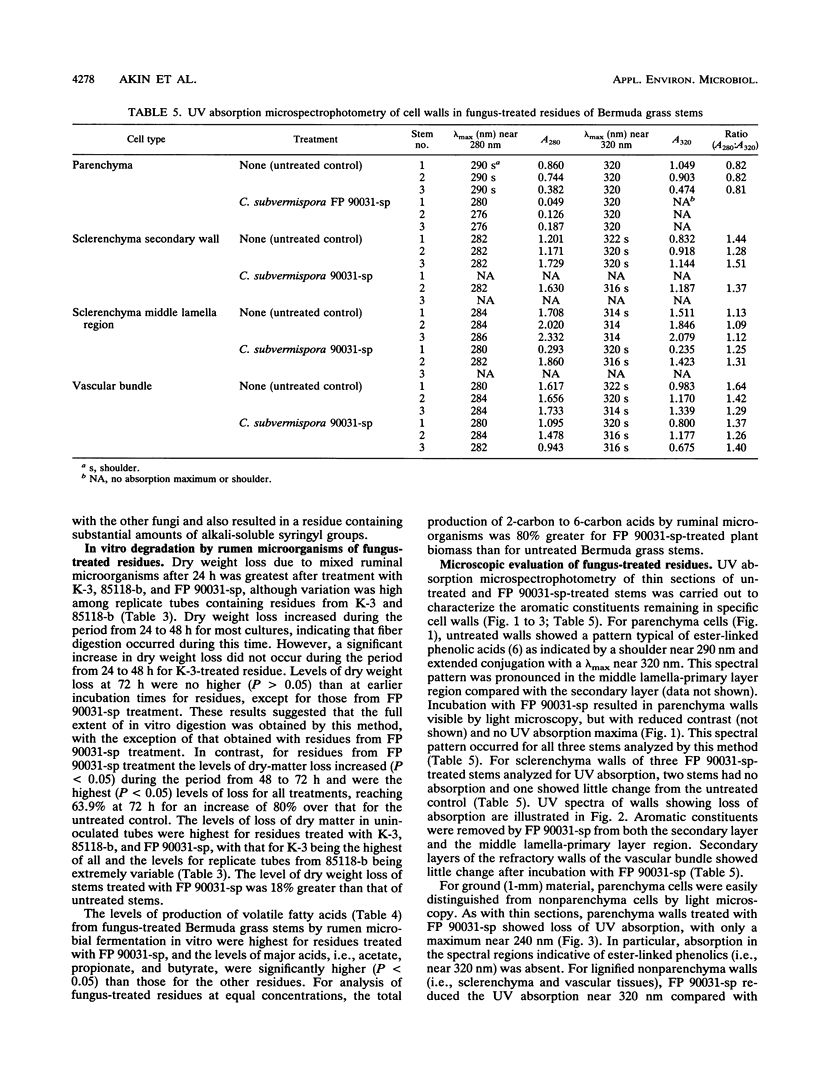
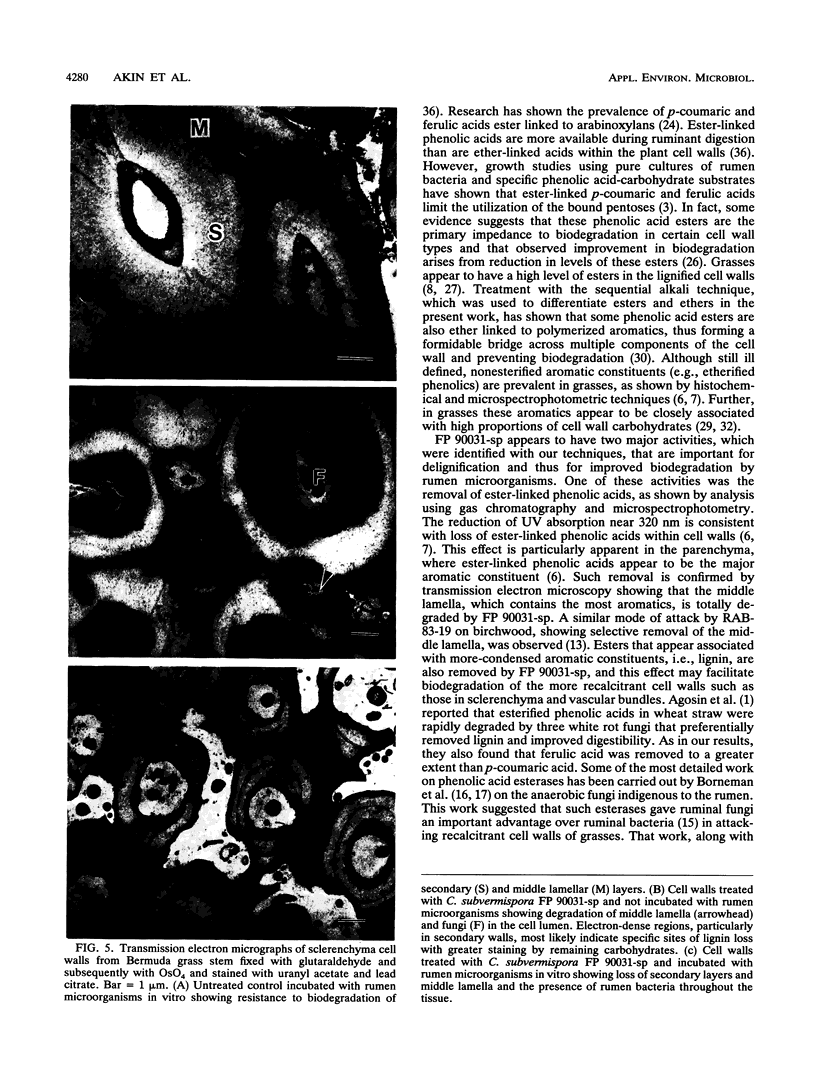
Three wild-type white rot fungi and two cellulase-less mutants developed from Phanerochaete chrysosporium K-3 (formerly Sporotrichum pulverulentum) were tested for their ability to delignify grass cell walls and improve biodegradation by rumen microorganisms. Fungal-treated and control stems of Bermuda grass were analyzed for their content of ester- and ether-linked aromatics by using alkali extraction and gas chromatography, for in vitro dry weight digestion and production of volatile fatty acids in in vitro fermentations with mixed ruminal microorganisms, for loss of lignin and other aromatics from specific cell wall types by using microspectrophotometry, and for structural changes before and after in vitro degradation by rumen microorganisms by using transmission electron microscopy. P. chrysosporium K-3 and Ceriporiopsis subvermispora FP 90031-sp produced the greatest losses in lignin and improved the biodegradation of Bermuda grass over that of untreated control substrate. However, C. subvermispora removed the most lignin and significantly improved biodegradation over all other treatments. Phellinus pini RAB-83-19 and cellulase-less mutants 3113 and 85118 developed from P. chrysosporium K-3 did not improve the biodegradation of Bermuda grass lignocellulose. Results indicated that C. subvermispora extensively removed ester-linked p-coumaric and ferulic acids and also removed the greatest amount of non-ester-linked aromatics from plant cell walls. Microscopic observations further indicated that C. subvermispora removed esters from parenchyma cell walls as well as esters and lignin from the more recalcitrant cell walls (i.e., sclerenchyma and vascular tissues). C. subvermispora improved in vitro digestion and volatile fatty acid production by ruminal microorganisms by about 80%, while dry matter loss due to fungi was about 20% greater than loss in untreated control stems. The chemical and structural studies used identified sites of specific fungal attack and suggested mechanisms whereby improvement occurred.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Borneman W. S., Rigsby L. L., Martin S. A. p-Coumaroyl and feruloyl arabinoxylans from plant cell walls as substrates for ruminal bacteria. Appl Environ Microbiol. 1993 Feb;59(2):644–647. doi: 10.1128/aem.59.2.644-647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette R. A., Reid I. D. Ultrastructural Aspects of Wood Delignification by Phlebia (Merulius) tremellosus. Appl Environ Microbiol. 1986 Aug;52(2):239–245. doi: 10.1128/aem.52.2.239-245.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette R. A. Screening wood decayed by white rot fungi for preferential lignin degradation. Appl Environ Microbiol. 1984 Sep;48(3):647–653. doi: 10.1128/aem.48.3.647-653.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman W. S., Hartley R. D., Himmelsbach D. S., Ljungdahl L. G. Assay for trans-p-coumaroyl esterase using a specific substrate from plant cell walls. Anal Biochem. 1990 Oct;190(1):129–133. doi: 10.1016/0003-2697(90)90145-y. [DOI] [PubMed] [Google Scholar]
- Borneman W. S., Ljungdahl L. G., Hartley R. D., Akin D. E. Purification and partial characterization of two feruloyl esterases from the anaerobic fungus Neocallimastix strain MC-2. Appl Environ Microbiol. 1992 Nov;58(11):3762–3766. doi: 10.1128/aem.58.11.3762-3766.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle C. D., Kropp B. R., Reid I. D. Solubilization and mineralization of lignin by white rot fungi. Appl Environ Microbiol. 1992 Oct;58(10):3217–3224. doi: 10.1128/aem.58.10.3217-3224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. G., Valdez F. R., Abad A. R., Blanchette R. A., Hatfield R. D. Effect of white rot basidiomycetes on chemical composition and in vitro digestibility of oat straw and alfalfa stems. J Anim Sci. 1992 Jun;70(6):1928–1935. doi: 10.2527/1992.7061928x. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Nisbet D. J. Effects of Aspergillus oryzae fermentation extract on fermentation of amino acids, bermudagrass and starch by mixed ruminal microorganisms in vitro. J Anim Sci. 1990 Jul;68(7):2142–2149. doi: 10.2527/1990.6872142x. [DOI] [PubMed] [Google Scholar]
- Rüttimann-Johnson C., Salas L., Vicuña R., Kirk T. K. Extracellular Enzyme Production and Synthetic Lignin Mineralization by Ceriporiopsis subvermispora. Appl Environ Microbiol. 1993 Jun;59(6):1792–1797. doi: 10.1128/aem.59.6.1792-1797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]