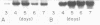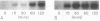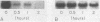Abstract
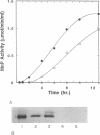
The expression of manganese peroxidase (MnP) in nitrogen-limited cultures of Phanerochaete chrysosporium is regulated by heat shock at the level of gene transcription. Nitrogen limitation and manganous ion [Mn(II)] previously have been shown to regulate mnp gene transcription. Northern (RNA) blot analysis demonstrates that 45°C heat shock results in the accumulation of mnp mRNA, even in cells grown in the absence of Mn. Heat shock induces mnp gene transcription in 4- or 5-day-old cells, and mnp mRNA is detectable after 15 min at 45°C. Maximum accumulation of mnp mRNA is observed 1 to 2 h after transfer of cultures to 45°C. Two hours after heat shock-induced cultures grown in the absence of Mn are transferred back to 37°C, mnp mRNA is no longer detectable. Higher levels of mnp mRNA are obtained with simultaneous induction by Mn and heat shock than by either treatment alone. Neither MnP enzyme activity nor protein is detectable in heat-shocked cultures grown in the absence of Mn. However, higher MnP activity is found in the extracellular medium of cultures induced by both heat shock and Mn than in the medium of cultures induced by Mn alone. These results suggest that the putative heat shock elements found in the promoter region of the mnp genes are physiologically functional and that Mn may be required for a posttranscriptional step of MnP production under heat shock conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alic M., Letzring C., Gold M. H. Mating System and Basidiospore Formation in the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1987 Jul;53(7):1464–1469. doi: 10.1128/aem.53.7.1464-1469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Alic M., Gold M. H. Manganese peroxidase gene transcription in Phanerochaete chrysosporium: activation by manganese. J Bacteriol. 1991 Jul;173(13):4101–4106. doi: 10.1128/jb.173.13.4101-4106.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Glenn J. K., Gold M. H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990 Jun;172(6):3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta V. C., Hamer D. H. Fine mapping of a mouse metallothionein gene metal response element. Mol Cell Biol. 1989 Mar;9(3):1376–1380. doi: 10.1128/mcb.9.3.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Godfrey B. J., Mayfield M. B., Brown J. A., Gold M. H. Characterization of a gene encoding a manganese peroxidase from Phanerochaete chrysosporium. Gene. 1990 Sep 1;93(1):119–124. doi: 10.1016/0378-1119(90)90144-g. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Alic M. Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol Rev. 1993 Sep;57(3):605–622. doi: 10.1128/mr.57.3.605-622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. H., Cheng T. M. Induction of colonial growth and replica plating of the white rot basidiomycete Phanaerochaete chrysosporium. Appl Environ Microbiol. 1978 Jun;35(6):1223–1225. doi: 10.1128/aem.35.6.1223-1225.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. M., Larin Z., Taylor I. C., Prentice H., Gwinn K. A., Kingston R. E. Multiple basal elements of a human hsp70 promoter function differently in human and rodent cell lines. Mol Cell Biol. 1987 Oct;7(10):3646–3655. doi: 10.1128/mcb.7.10.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Fürst P., Hamer D. The DNA and Cu binding functions of ACE1 are interdigitated within a single domain. New Biol. 1990 Jun;2(6):544–555. [PubMed] [Google Scholar]
- Imbert J., Culotta V., Fürst P., Gedamu L., Hamer D. Regulation of metallothionein gene transcription by metals. Adv Inorg Biochem. 1990;8:139–164. [PubMed] [Google Scholar]
- Kapoor M., Lewis J. Heat shock induces peroxidase activity in Neurospora crassa and confers tolerance toward oxidative stress. Biochem Biophys Res Commun. 1987 Sep 30;147(3):904–910. doi: 10.1016/s0006-291x(87)80156-0. [DOI] [PubMed] [Google Scholar]
- Kapoor M., Sreenivasan G. M., Goel N., Lewis J. Development of thermotolerance in Neurospora crassa by heat shock and other stresses eliciting peroxidase induction. J Bacteriol. 1990 May;172(5):2798–2801. doi: 10.1128/jb.172.5.2798-2801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993 Mar 5;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Müller R. M., Taguchi H., Shibahara S. Nucleotide sequence and organization of the rat heme oxygenase gene. J Biol Chem. 1987 May 15;262(14):6795–6802. [PubMed] [Google Scholar]
- Pease E. A., Andrawis A., Tien M. Manganese-dependent peroxidase from Phanerochaete chrysosporium. Primary structure deduced from cDNA sequence. J Biol Chem. 1989 Aug 15;264(23):13531–13535. [PubMed] [Google Scholar]
- Pease E. A., Tien M. Heterogeneity and regulation of manganese peroxidases from Phanerochaete chrysosporium. J Bacteriol. 1992 Jun;174(11):3532–3540. doi: 10.1128/jb.174.11.3532-3540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D., Mayfield M. B., Nipper V. J., Brown J. A., Gold M. H. Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem. 1989 Mar 25;264(9):5036–5040. [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston D. M., O'Halloran T. V. Metalloregulatory proteins and molecular mechanisms of heavy metal signal transduction. Adv Inorg Biochem. 1990;8:1–31. [PubMed] [Google Scholar]
- Silar P., Butler G., Thiele D. J. Heat shock transcription factor activates transcription of the yeast metallothionein gene. Mol Cell Biol. 1991 Mar;11(3):1232–1238. doi: 10.1128/mcb.11.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Tien M., Tu C. P. Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature. 1987 Apr 2;326(6112):520–523. doi: 10.1038/326520a0. [DOI] [PubMed] [Google Scholar]
- Tuor U., Wariishi H., Schoemaker H. E., Gold M. H. Oxidation of phenolic arylglycerol beta-aryl ether lignin model compounds by manganese peroxidase from Phanerochaete chrysosporium: oxidative cleavage of an alpha-carbonyl model compound. Biochemistry. 1992 Jun 2;31(21):4986–4995. doi: 10.1021/bi00136a011. [DOI] [PubMed] [Google Scholar]
- Valli K., Brock B. J., Joshi D. K., Gold M. H. Degradation of 2,4-dinitrotoluene by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Jan;58(1):221–228. doi: 10.1128/aem.58.1.221-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Gold M. H. Degradation of 2,4-dichlorophenol by the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol. 1991 Jan;173(1):345–352. doi: 10.1128/jb.173.1.345-352.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Wariishi H., Gold M. H. Degradation of 2,7-dichlorodibenzo-p-dioxin by the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1992 Apr;174(7):2131–2137. doi: 10.1128/jb.174.7.2131-2137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wariishi H., Akileswaran L., Gold M. H. Manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: spectral characterization of the oxidized states and the catalytic cycle. Biochemistry. 1988 Jul 12;27(14):5365–5370. doi: 10.1021/bi00414a061. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Dunford H. B., MacDonald I. D., Gold M. H. Manganese peroxidase from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Transient state kinetics and reaction mechanism. J Biol Chem. 1989 Feb 25;264(6):3335–3340. [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J Biol Chem. 1992 Nov 25;267(33):23688–23695. [PubMed] [Google Scholar]