Abstract
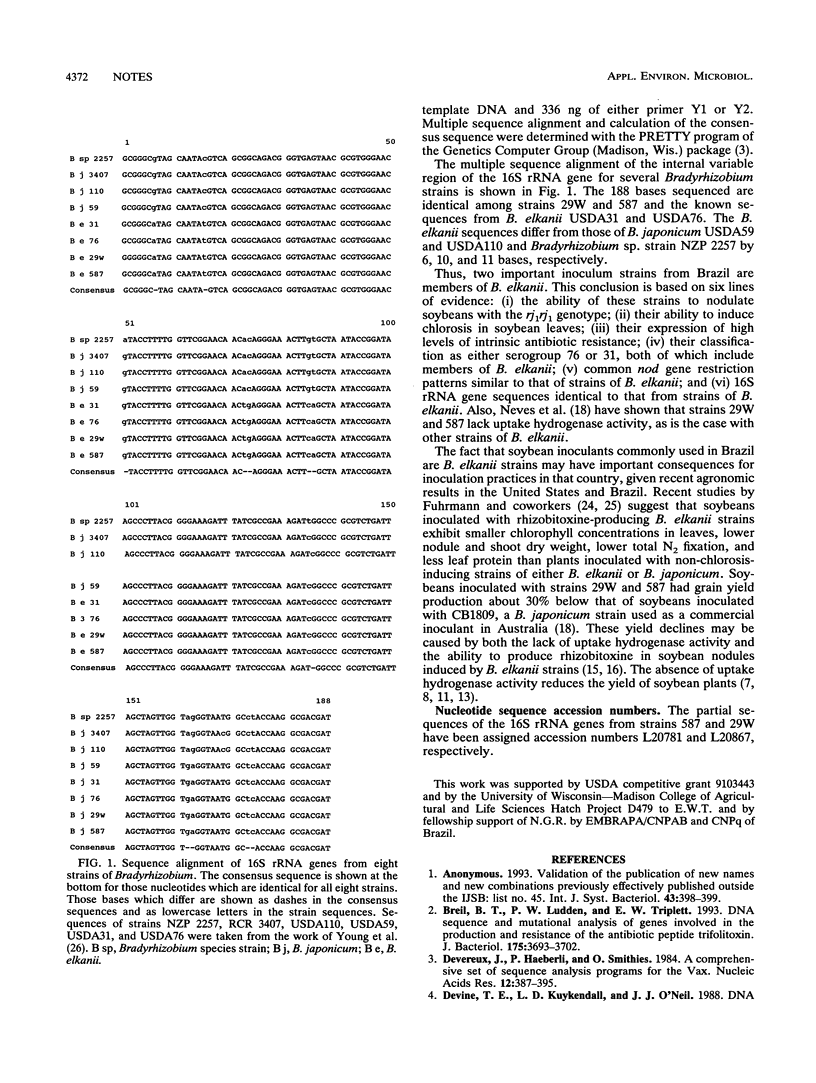
The Brazilian inoculant strains 29W and 587 were found to be members of Bradyrhizobium elkanii primarily on the basis of 16S rRNA gene sequences identical to that of B. elkanii USDA76 and on the basis of reactivity with antibodies against serogroups 76 and 31, respectively. The agronomic consequences of using strains of B. elkanii as soybean inoculants are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breil B. T., Ludden P. W., Triplett E. W. DNA sequence and mutational analysis of genes involved in the production and resistance of the antibiotic peptide trifolitoxin. J Bacteriol. 1993 Jun;175(12):3693–3702. doi: 10.1128/jb.175.12.3693-3702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Harker A. R., Papen H., Russell S. A., Hanus F. J., Zuber M. Physiology, biochemistry, and genetics of the uptake hydrogenase in rhizobia. Annu Rev Microbiol. 1987;41:335–361. doi: 10.1146/annurev.mi.41.100187.002003. [DOI] [PubMed] [Google Scholar]
- Fuhrmann J. Symbiotic effectiveness of indigenous soybean bradyrhizobia as related to serological, morphological, rhizobitoxine, and hydrogenase phenotypes. Appl Environ Microbiol. 1990 Jan;56(1):224–229. doi: 10.1128/aem.56.1.224-229.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann J., Wollum A. G. Simplified Enzyme-Linked Immunosorbent Assay for Routine Identification of Rhizobium japonicum Antigens. Appl Environ Microbiol. 1985 Apr;49(4):1010–1013. doi: 10.1128/aem.49.4.1010-1013.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Production of the Soybean-Chlorosis Toxin by Rhizobium japonicum in Pure Culture. Plant Physiol. 1965 Sep;40(5):931–933. doi: 10.1104/pp.40.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Cregan P. B., Keyser H. H. DNA Hybridization Probe for Use in Determining Restricted Nodulation among Bradyrhizobium japonicum Serocluster 123 Field Isolates. Appl Environ Microbiol. 1990 Jun;56(6):1768–1774. doi: 10.1128/aem.56.6.1768-1774.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Young J. P., Downer H. L., Eardly B. D. Phylogeny of the phototrophic rhizobium strain BTAi1 by polymerase chain reaction-based sequencing of a 16S rRNA gene segment. J Bacteriol. 1991 Apr;173(7):2271–2277. doi: 10.1128/jb.173.7.2271-2277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


