Abstract
Objective
To offer a physics course that is relevant to pharmacy students, yet still contains many of the fundamental principles of physics.
Design
The course was modified over a period of several years to include activities and examples that were related to other courses in the curriculum.
Assessment
Course evaluations were given to assess student attitudes about the importance of physics in the pharmacy curriculum.
Conclusion
Students' attitudes have changed over time to appreciate the role that physics plays in their studies. Students gained confidence in their ability to learn in other courses.
Keywords: physical science, physics, health-related applications, prepharmacy curriculum
INTRODUCTION
Science courses are an important part of the curriculum at most colleges of pharmacy. Courses such as biology, anatomy, physiology, general chemistry, organic chemistry, calculus, and physics are included in the prepharmacy curriculum. In 1993 the American Association of Colleges of Pharmacy (AACP) reported in a series of papers that a pharmaceutical education includes a study of the physical and biological sciences.1-3 The American Council on Pharmaceutical Education (ACPE) has set guidelines for preprofessional requirements for admission to the professional program that include “basic sciences, such as general chemistry, organic chemistry, biological sciences (with a focus on human processes and diseases), mathematics, information and communication technologies, and physical sciences.”4
The close connection of pharmacy to the sciences is observed in the AACP's Center for the Advancement of Pharmaceutical Education (CAPE) 2004 Educational Outcomes and Supplements.5-12 Three terminal ability outcomes for pharmacy graduates are listed: pharmaceutical care, systems management, and public health. For the pharmaceutical care outcome, discipline-specific outcomes are listed in the supplementary documents. For example, graduates are to “utilize and integrate knowledge of physiology, pathophysiology, and anatomy in order to formulate a therapeutic care plan.”7 Other outcomes refer to application of biology, medicinal chemistry, pharmacokinetics, and pharmacology to provide patient-specific and evidence-based pharmaceutical care.
Physicists often refer to their subject as a foundational course for understanding the other sciences. However, in the pharmaceutical education reports mentioned above, there is a shift away from the term “physics” to a more generic term “physical sciences.” In 2000, the author reported results of a survey that was administered to determine how colleges of pharmacy have interpreted these guidelines regarding the inclusion of physics in the prepharmacy curriculum.13 To summarize the results, roughly three fourths of the colleges require physics, and of those colleges, just over half require a full year of physics, while the remaining require only 1 semester. Eley and Parsons argued for the inclusion of physics in the prepharmacy curriculum.14,15
At the St. Louis College of Pharmacy, students take all 6 years of a curriculum that leads to the PharmD degree, including the 2 years generally associated with a preprofessional curriculum. Because the only major at the college is pharmacy, nonpharmacy faculty members often seek to relate their course material to the curriculum so as to emphasize its relevance to students. In particular, students often fail to see the connection between physics and their other courses, especially to their studies in pharmacy, but also to those they consider more applicable, such as chemistry, anatomy, and physiology. Part of a faculty member's/instructor's responsibility is to make these connections. This paper describes how one faculty member/instructor changed the physics course to make it more relevant. Two specific physics topics will be described in greater detail, along with a discussion of outcomes of the course, and a final summary.
DESIGN
The physics course taught at the St. Louis College of Pharmacy has changed over time. For several years, the course was similar to physics courses taught at most colleges and universities, which had few examples and applications to the human body or to the medical sciences.
Changing the course involved a number of approaches. The instructor had to be flexible enough to realize that a typical physics course was not going to go over well. Examples and applications appropriate for students in the 18- to 20-year old range were developed for the course. From 1997 through 2000, the instructor attended several workshops and conferences that discussed applications of physics to the human body. Information gathered at these workshops aided the instructor in selecting an appropriate textbook for the course.16,17 Major revisions of the course were completed for the fall semester of 2000. At the end of each semester, students completed a standard course evaluation. Feedback from these evaluations was used to make additional, but small, changes to the course over the last few years. The rest of this section describes the course in greater detail.
Students were required to take a 1-semester, 4-credit-hour physics course. Three ability outcomes are listed in the course syllabus:
Thinking and decision-making: students are to collect information from a variety of sources, organize the information appropriately, understand and use the information correctly, analyze problems to determine important and unimportant information, and recognize misconceptions and take steps to correct them.
Scientific competence: students are to study a variety of physical phenomena, recognize basic principles and concepts underlying these phenomena, develop mathematical models that describe the phenomena, and apply these models to evaluate information.
Mathematical reasoning: students are to understand quantitative relationships, perform necessary mathematical operations, and determine the reasonableness of a quantitative result.
These outcomes are aligned with the college's ability outcomes and were written specifically for the physics course. Emphasis is placed on learning, thinking, analyzing, and reasoning—all of which are important skills for the pharmacy student and graduate.
The course covers most of the topics taught in a typical physics course, but with special emphasis on applications to the human body and to other biomedical science courses. In addition, references are made to courses like pharmaceutics, pharmacokinetics, and evaluation of research studies. Examples of topics covered include: classical mechanics and motion of the human body; fluid statics and dynamics related to pressure, blood flow, and intravenous fluids18; thermodynamics related to body temperature, fever, thermal conductivity of skin, food calories, and heat capacity; wave properties including sound production by the vocal chords, sound detection by the ear, sound intensity level, and the use of earplugs; electrostatics related to membrane potential and action potential; optics of the eye, with emphasis on vision problems and correction techniques, including reading glasses; and nuclear decay, including biological effects of radiation and use of radiopharmaceuticals.
The following sections describe in more detail 2 topics covered in the course: fluids and nuclear decay. Both topics are important for developing ability outcomes listed in the CAPE 2004 Educational Outcomes Supplements, such as describing effective routes of drug administration, particularly through the circulatory system, or selecting optimal therapeutic agents, such as radioisotopes for treatment of a cancerous tumor.9-12
The Physics of Fluids
Because the physics of fluids is quite complex, students went through basic concepts related to fluids at rest and fluids in motion with a goal to see how they apply to the human body. The concept of pressure, often described simply as force per area, was discussed in terms of 3 causes: pressure due to the weight of a fluid, pressure due to an external force on an enclosed fluid, and pressure due to the interaction of molecules with themselves and their container.
The first cause has to do with the fact that below the surface of a fluid, the pressure increases with depth because the weight of the fluid produces a force on the surface of a submerged object. This concept explains why pressure on a person's ears increases when the individual dives to the bottom of a pool of water. It also arises when discussing atmospheric pressure, which is due to the “weight” of air at the surface of the earth, or at heights of 33,000 feet above the surface in an airplane. Why do people who work on their feet have swollen ankles? In class students worked through an example that showed that blood pressure in the ankle can be as much as 100 mm-Hg or more above normal blood pressure. The equation used is
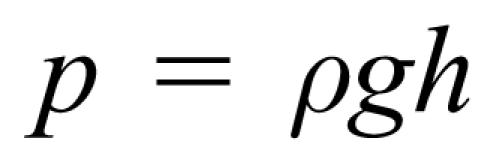 |
where p is the pressure, ρ is the density of blood, g is the acceleration due to gravity, and h is the distance from the level of the heart to the foot. Close attention was paid to using proper units, including the International System of Units (or SI, from the French Le Système International d'Unités) and common units.
The second cause of pressure in fluids is due to an external force applied to an enclosed container of fluid. A simple example is when one squeezes on a balloon, the pressure in the balloon goes up, perhaps enough to cause it to burst. What happens to the pressure in the lungs when someone is about to cough? The diaphragm is forced upward to press against the space that includes the lungs while the throat closes off the path of escape; this action causes pressure to increase significantly. Blood transfusions or injections of medication occur more quickly when external pressure is applied to the bag or when a force is applied to the plunger of a syringe.
The third cause of pressure arises from the interaction of molecules with themselves or their container as they move and collide with each other. This cause primarily involves gases, and a discussion of the various gas laws is included. Once again, an application to the lungs is considered, as well as to the contraction and relaxation of the heart as it goes through its normal rhythm.
Other examples of pressure and the human body include: the bladder when full and empty, with application to an enlarged prostate and difficulty in urination; the eyeball, including glaucoma and how medication works to relieve pressure; the brain with reference to hydrocephalus and medical treatment; and bones and joints, with a brief discussion of bone density and osteoporosis.
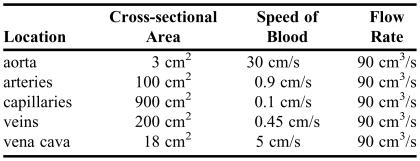
Another major concept is the motion of fluids, which is called fluid dynamics. Students learned about flow rate, defined as the volume of fluid flow per time, and viscosity, which requires that continuous pressure be applied to move the fluid because of energy losses. Application to blood flow in the circulatory system, both the systemic and pulmonary circuits, is discussed. Calculations were done to show how fast blood flows in the aorta and how slowly it flows in capillaries. The equation used is called the flow rate equation:
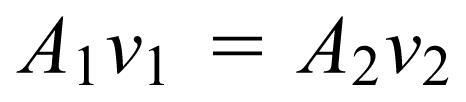 |
where A 1 and A 2 are the cross-sectional areas of 2 different sections of the system, and v 1 and v 2 represent the speed of the blood in those sections. The equation assumes that the volume of blood flowing in the circulatory system is constant over a relatively short amount of time. Table 1 compares the cross-sectional area, the speed of blood, and the flow rate at various positions along the circulatory system. (The numbers shown in the table are for illustrative purposes only and are not intended to represent values in all individuals.) Students observed that the physics of blood flow is related directly to the physiology of blood flow, such as the exchange of gases in the capillaries and the optimal shape of the various chambers of the heart.
Table 1.
Comparison of Blood Flow Along the Systemic Circulatory System
Students were presented with an example in which a patient is to be given a blood transfusion. Blood should flow from a bag hanging on a pole and then through a tube connected to a needle inserted into a vein in the arm. (Students were asked “Why a vein and not an artery?”) Many important concepts converge in this problem, including pressure variation with depth, volume flow rate, and Poiseuille's law. Poiseuille's law, represented by the equation
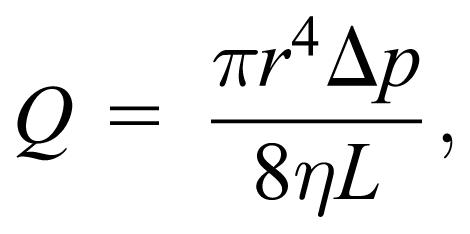 |
relates the flow rate Q to physical dimensions of the needle (radius r and length L), the pressure difference along the needle Δp, and the viscosity of blood η. An example problem, which students approached, involves a patient who is to be given 500 cc of blood in 30 minutes. By the end of the unit, students should be able to show that for a certain needle size and if the pressure in the vein is assumed to be zero, then the height of the bag must be about 0.5 m above the needle in the arm. If a nonzero value for the pressure in the arm is assumed (eg, 20 mm Hg), then it can be shown that the height of the bag should be about 1 m above the needle. Students learned that the needle is the rate-limiting factor in the problem, so that the relatively large tube from the bag to the needle has little effect on the flow rate. Variations on this problem were made that take into account different needle sizes, different fluids or viscosities, and differences in the position of the patient or the location of the needle.
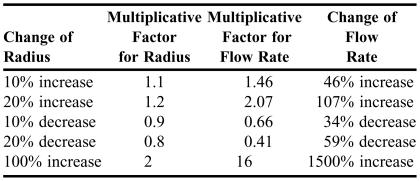
Another example was used that explored the dependence of flow rate in Poiseuille's law on the radius of a tube, which is raised to the fourth power. This dependence is very strong: it means that if the radius of a tube were to double, then if all other parameters remain the same, the flow rate would increase by a multiplicative factor of 16. Related to the human body, it can be shown from purely physical principles that if the radius of a clogged coronary artery were to increase by only 20%, then the flow rate would increase by over 100%. Table 2 shows in more detail this strong dependence. Students were asked about ways to increase the radius of a clogged artery; such as bypass surgery, balloon angioplasty, a stint, or medication. Students use this information later in their coursework.
Table 2.
Examples of Strong Dependence of Flow Rate on the Radius of a Tube Given by Poiseuille's Law
Students also learned that the concept of pressure can be applied to solids as well. Examples of bone mass density measurements related to osteoporosis and osteopenia were discussed. Other courses where this concept is important include pharmaceutics and dosage forms such as tablet making and compounding.
Nuclear Decay
Nuclear physics is a large field of study in the physics discipline, but it draws little attention in the other sciences. However, nuclear medicine plays a major role in the health industry, mainly in diagnostic and therapeutic applications. Knowledge of basic nuclear physics principles is important for students wishing to pursue careers in this area. Major topics covered in the physics course include fundamental concepts such as isotopes, types of nuclear radiation, and mathematics of nuclear decay (decay rate, half-life, etc), as well as applications to health and medicine, such as units of dosage, diagnostic techniques, and therapeutic procedures.
Students learned about the make-up of the nucleus, the strong nuclear force that holds protons and neutrons together, and nuclear notation, such as carbon-12, 12C, or polonium-210, 210Po. They also learned about isotopes of elements, which have the same number of protons, but different numbers of neutrons, such as 12C and 14C, or 128I or 131I. They learned that isotopes of an element have the same chemical properties and bind to other compounds in the same way, and that certain isotopes have stable nuclei and others are unstable, which makes them radioactive.
This radiation is usually 1 of 3 types: alpha, beta, or gamma radiation. Other types of nuclear processes are discussed, such as neutron emission or electron capture. Terms such as parent nucleus and daughter nucleus are defined. Nuclear decay equations (similar to chemical reaction equations) are described, in which students learn that the total number of nucleons (neutrons and protons) is conserved when a parent nucleus decays to a daughter nucleus giving off radiation. An example of a nuclear decay equation involving alpha decay is
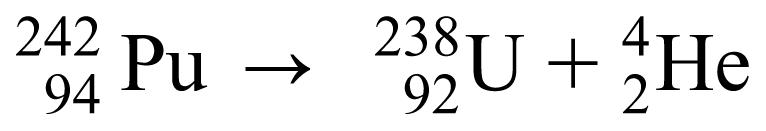 |
In this example, the parent nucleus, plutonium (Pu) with 94 protons and 242 nucleons, decays to the daughter nucleus, uranium (U), with 92 protons and 238 nucleons, and gives off an alpha particle, which is identical to the nucleus of a helium (He) atom with 2 protons and 4 nucleons. Another example, technetium-99m, gives off gamma radiation with the following decay equation:
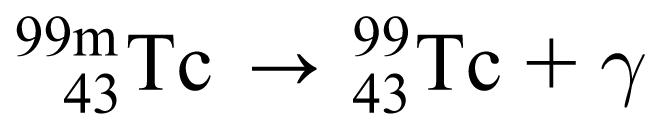 |
where the letter “m” stands for “metastable,” the excited state form of technetium.
The mathematics of nuclear decay involves several different parameters. Decay rate, or activity, is a measure of the number of decays per time. Units of decay rate are the curie (Ci) and the becquerel (Bq). The half-life of an isotope is the time for the population of the radioactive material to decrease by one-half. Half-lives of various isotopes range from less than a femtosecond (10−15 s) to billions of years. The exponential behavior of radioactive decay applies to the number of parent nuclei in a radioactive sample, as well as to the mass of the sample and to the activity of the sample. The exponential equation written in terms of activity R(t) as a function of time is
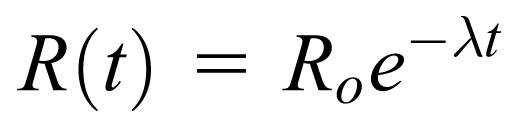 |
where R o is the initial activity when first measured (at t = 0) and λ is the decay constant, which is dependent on the radioactive half-life of the sample. The exponential portion of the equation is sometimes referred to in the medical literature as the “fraction remaining” of the radioactive sample because it is a number less than one.
When discussing the biological effects of radiation, the 3 major types of radiation are compared. Students learned that alpha particles are massive and move slowly, but actually do the most damage to biological tissue because they are easily absorbed, usually within a fraction of a millimeter in the skin. Beta particles, including electrons (beta minus) and positrons (beta plus or antielectrons) have much less mass, move rapidly, and do not cause as much damage because they are not as easily absorbed as alpha particles. Gamma particles are electromagnetic waves (or photons or rays) that move at the speed of light and are not easily absorbed, and do little damage. Even though beta particles and gamma particles are not easily absorbed, it does not mean that they do no damage. Any radiation (sometimes called ionizing radiation) absorbed in human tissue is capable of causing damage. The fact that damage to human tissue can occur means that radiation can cause cancer or it can be used to treat cancer.
The recent, well-publicized, death of a former Russian spy brings up a good case study for understanding these concepts. To summarize, the individual was “poisoned” by ingesting radioactive 210Po (polonium), which decays by emitting an alpha particle. Because alpha particles are easily absorbed, exposure from outside of his body likely would have caused serious damage to skin, but the damage would have been limited to outer tissues. However, because the material was sprinkled on his food, his stomach and other organs were exposed, which caused enough tissue damage to kill him.
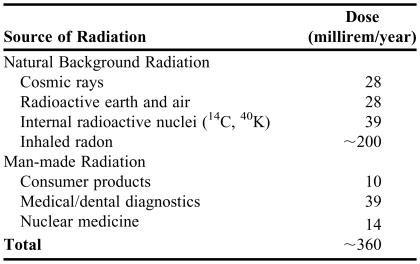
The amount of radiation absorbed by human tissue is described in 2 similar, but different, ways. The first method is called “dose” and is related to how much energy per mass is deposited when the radiation is absorbed. The common unit is the “rad,” which is equal to 0.01 J/kg. The International System of Units (SI) for dose is the gray (Gy) where 1 gray = 100 rad. Because different types of radiation cause different amounts of damage, it turns out that 1 rad of alpha radiation does about 20 times more damage than 1 rad of gamma radiation.16 The second method of measuring the amount of radiation absorbed is called “effective dose” and takes these differences into account. The typical unit is the “rem” and the SI unit is the sievert (Sv). Thus, 1 rem of any type radiation does about the same amount of damage to human tissue. Students learned about maximum exposure values (5 rem per year or 3 rem over a 3 month period) and typical exposure values of US citizens, which is about 400 millirem per year, and sources of radiation to which typical US citizens are exposed.16,19,20 Roughly half of this amount comes from inhaled radon gas, which varies widely with geographical regions. Table 3 gives more specific values of the sources of radiation.19,20
Table 3.
After learning about physical concepts of nuclear radiation, students looked at medical applications, particularly therapeutic and diagnostic techniques. Therapeutic techniques include the following:
Cancer treatment using external devices that produce x-ray or gamma rays, such as 60Co (cobalt) sources, Gamma Knife, and CyberKnife. The deep penetration of this type of radiation allows treatment below the surface of the skin, such as brain tumors, etc.
Cancer treatment using internal devices that are beta particle emitters. This procedure is called brachytherapy where the radioactive material is embedded in small objects called seeds, or ribbons, or beads. These objects are implanted into or near the tumor because beta particles are more easily absorbed than gamma rays.21
Radioimmunotherapy (RIT) using beta emitters such as 90Y (yttrium) to treat non-Hodgkin's lymphoma and 131I (iodine) to treat thyroid cancer.21,22
Palliative agents such as 98Sr (strontium) or 153Sm (samarium) to relieve pain due to bone disease.21,22
Intravascular therapy, which uses radiation from a radioisotope embedded in a catheter. The radiation exposes plaque in the coronary arteries to help prevent them from reclosing.21
Diagnostic applications primarily involve radiopharmaceuticals that are taken up by a target organ or system and emit radiation that is imaged by various instruments using a process called tomography. Examples of techniques include the following:
Single Photon Emission Computed Tomography (SPECT), which measures gamma radiation
Positron Emission Tomography (PET), which measures positron (beta-plus) radiation
X-ray detectors that measure x-rays emitted after electron capture
Electron detectors
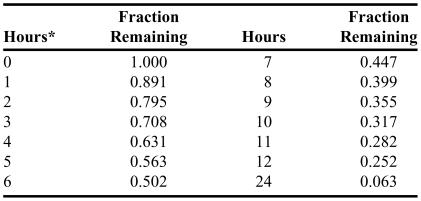
The author used a personal experience with the health care system—undergoing a nuclear stress test using a MYOVIEW kit—as an example that brought all of these topics together. The procedure involved injection of 99mTc (technetium) Tetrofosmin, which is a gamma emitter. A SPECT machine was used to determine proper flow of blood in the coronary arteries. The information sheet that comes with the kit includes a description of the physical characteristics of radioactive technetium, such as the energy of the gamma particles and the effectiveness of lead shielding, as well as decay charts that list the fraction of remaining material as a function of time based on the half-life of 99mTc.23
Table 4 lists the decay chart given in the MYOVIEW information sheet. As an example to help illustrate the usefulness of the chart, suppose a patient is injected with 0.23 mg of Tetrofosmin; after 2 hours time has elapsed there should be about 0.795 × 0.23 mg = 0.18 mg of radioactive Tetrofosmin remaining in the patient, provided no other path of elimination from the patient has occurred in that time. For times greater than 12 hours, the fraction remaining can be calculated from the other values given. For example, to determine the fraction remaining at 16 hours, the fraction at 12 hours is multiplied by the fraction at 4 hours: 0.252 × 0.631 = 0.159.
Table 4.
Decay Chart for Technetium-99m from a MYOVIEW Kit23
*Calibration time measured from the time of preparation
Students taking courses later on in the curriculum will find this information helpful. In particular, the nuclear pharmacy course looks at specific applications, using the background information learned in this course. Mathematical competence gained from these studies is helpful in pharmcokinetics.
DISCUSSION
Before the instructor made significant changes to the physics course, student attitudes generally were negative because they saw the course as being difficult, not interesting, and not relevant to their other courses, particularly to their study of pharmacy. Through the author's efforts to make the course more relevant, student attitudes have improved with time. On course evaluations forms, students indicated the relevance of physics to their studies. Responses included the following:
“Overall, I thought the course was very interesting.”
“It is a good class overall. It made physics easy to understand and to apply math (to) real life situations.”
“The (instructor) is a very good and tries his best to make the material more understandable by bringing in examples over the course study.”
“I think it was an excellent course … and I learned a lot from it. I even used some of the material to understand chemistry better …”
“I feel that the course was very thorough and I learned a lot of material that I can apply later in my life.”
“I do not really like physics. Lecture material was interesting. Radiation and its medical uses interested me.”
“I like that the course had Rx aspects.”
However, negative comments still occasionally arise such as the following quote, “This class is way too hard for something that pertains not the least bit to our degree. Give it up. No one cares about your … class.” Unfortunately, not everyone is won over.
References to medical applications of physics outside the classroom have occurred as well. Recently, when evaluating a research paper in Clinical Epidemiology, which students take 3 years after taking physics, students were able to recall the strong dependence of rate of fluid flow on the radius of a tube and apply this knowledge to the use of medication to obtain greater blood flow in patients with coronary artery disease (Gaebelein, C. private communication, August 24, 2006).
In addition, the author was invited to give a continuing education program on health aspects of nuclear physics and received positive feedback from participants.24 The program was similar in content to that presented in the physics course. Participants reported that the program was effective in enabling them to define common terminology of nuclear medicine, to explain the difference between a rad and a rem and between a curie and a becquerel, to state the safe dosage levels of radiation, and to describe how radiation is used in health-related applications.
SUMMARY
The prepharmacy curriculum includes courses that help students gain skills, knowledge, and attitudes that will enable them to succeed in the professional curriculum, particularly in the areas of problem solving and critical thinking. Physics is an important component of that curriculum. Students are motivated more strongly when their physics course is seen as relevant to their lives, especially to their study of pharmacy and other biomedical sciences. Hopefully, they will be able to see applications of these concepts in their other courses and as professional pharmacists.
REFERENCES
- 1.Commission to Implement Change in Pharmaceutical Education. Entry-level education in pharmacy: Commitment to change. Am J Pharm Educ. 1993;57:366–74. [Google Scholar]
- 2.Commission to Implement Change in Pharmaceutical Education. Background paper I: What is the mission of pharmaceutical education? Am J Pharm Educ. 1993;57:374–6. [Google Scholar]
- 3.Commission to Implement Change in Pharmaceutical Education. “Background paper II: Entry-level, curricular outcomes, curricular content and educational process,”. Am J Pharm Educ. 1993;57:377–85. [Google Scholar]
- 4. American Council on Pharmaceutical Education. Accreditation standards and guidelines for the professional program in pharmacy leading to the Doctor of Pharmacy degree. Available at: http://www.acpe-accredit.org/deans/standards.asp. Accessed December 8, 2006.
- 5. American Association of College of Pharmacy. Center for the Advancement of Pharmaceutical Education Educational Outcomes 2004. Available at: http://www.aacp.org/Docs/MainNavigation/Resources/6075_CAPE2004.pdf. Accessed December 15, 2006.
- 6. American Association of College of Pharmacy. 2004 CAPE Educational Outcomes Supplements. Available at: http://www.aacp.org/Docs/MainNavigation/Resources/7882_PREFACE-SummaryCAPE-December2006.pdf. Accessed December 15, 2006.
- 7. American Association of College of Pharmacy. 2004 CAPE Anatomy, Physiology, and Pathophysiology Supplemental Educational Outcomes. Available at: http://www.aacp.org/Docs/MainNavigation/Resources/7883_anatomyphysiologypathophysiologyDEC06.pdf. Accessed December 15, 2006.
- 8. American Association of College of Pharmacy. 2004 CAPE Biology Supplemental Educational Outcomes. Available at: http://www.aacp.org/Docs/MainNavigation/Resources/7884_BiologyDEC06.pdf. Accessed December 15, 2006.
- 9. American Association of College of Pharmacy. 2004 CAPE Medicinal Chemistry Supplemental Educational Outcomes. Available at: http://www.aacp.org/Docs/MainNavigation/Resources/7886_MedicinalChemistryDEC06.pdf. Accessed December 15, 2006.
- 10. American Association of College of Pharmacy. 2004 CAPE Pharmaceutics Supplemental Educational Outcomes. Available at: http://www.aacp.org/Docs/MainNavigation/Resources/7887_PharmaceuticsDEC06.pdf. Accessed December 15, 2006.
- 11. American Association of College of Pharmacy. 2004 CAPE Pharmacokinetics Supplemental Educational Outcomes. Available at: http://www.aacp.org/Docs/MainNavigation/Resources/7888_PharmacokineticsDEC06.pdf. Accessed December 15, 2006.
- 12. American Association of College of Pharmacy. 2004 CAPE Pharmacology Supplemental Educational Outcomes. Available at: http://www.aacp.org/Doc/MainNavigation/Resources/7889_PharmacologyDEC06.pdf. Accessed December 15, 2006.
- 13.McCall RP. Physics in the pre-pharmacy curriculum. Am J Pharm Educ. 2000;64:297–301. [Google Scholar]
- 14.Eley J. Need for maintaining basic sciences in the PharmD program. Am J Pharm Educ. 1999;63:253. [Google Scholar]
- 15.Parsons WH. Physics: its place in the pharmaceutical curriculum. Am J Pharm Educ. 1967;31:504–11. [Google Scholar]
- 16.Wilson JD, Buffa AJ, Lou B. College Physics. 6th ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2007. p. 925. [Google Scholar]
- 17. Several introductory physics textbooks have a good representation of biological applications. The author uses the textbook listed in Ref. 16. Others include: Jones E, Childers R. Contemporary College Physics. 4th ed. New York: McGraw–Hill; 2005; Urone PP. College Physics. 2nd ed. Pacific Grove, CA: Brooks/Cole; 2001; Walker JS. Physics. 3rd ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2007.
- 18.McCall RP. Physics and pharmacy: more than “ph.”. Phys Teach. 1998;36:408–9. [Google Scholar]
- 19. Tansil JE. Natural radioactivity in humans. Meeting of the Missouri Association of Physics Teachers, University of Missouri at Rolla, October 21, 2000.
- 20. National Council on Radiation Protection and Measurement. Ionizing Radiation Exposure of the Population of the United States, Report No. 93. 1987.
- 21.Coursey BM, Nath R. Radionuclide therapy. Phys Today. 2000;53:25–30. [Google Scholar]
- 22.Kowalsky RJ, Falen SW. Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine. Washington, DC: American Pharmaceutical Association; 2004. p. 498;768;779. [Google Scholar]
- 23. Amersham Health. MYOVIEW kit for the preparation of technetium Tc99m tetrofosmin for injection. Available at http://www.amershamhealth-us.com/shared/pdfs/pi/Myoview.pdf. Accessed December 8, 2006.
- 24. McCall RP. Biological effects of radiation, Continuing Education Program, St. Louis College of Pharmacy, St. Louis, Mo, October 13, 2006.