Abstract
The nicotine in tobacco is thought to modulate neuronal systems regulating mood. Moreover, it appears possible that blockade rather than activation of β2-containing (β2*) nicotinic acetylcholine receptors (nAChRs) may lead to antidepressant-like effects. We used cytisine, a partial agonist of α4/β2* nAChRs and a full agonist at α3/β4* nAChRs, in several tests of antidepressant efficacy. Further, we used c-fos expression to identify potential neurobiological correlates of the antidepressant-like effects of cytisine. Cytisine had antidepressant-like effects in several animal models of antidepressant efficacy. In addition, immunohistochemical analyses indicated that cytisine could reduce c-fos immunoreactivity in the basolateral amygdala by ~ 50%. These data show that cytisine acts like classical antidepressants in rodent models of antidepressant efficacy. In addition, cytisine’s ability to block α4/β2* nAChRs may be responsible for its antidepressant-like properties, and these may be mediated through a reduction of neuronal activity in the basolateral amygdala. These studies also suggest that both antagonists and partial agonists of α4/β2* nAChRs would be interesting targets for the development of novel antidepressant drugs.
Keywords: Nicotinic acetylcholine receptors, depression, nicotinic partial agonist, C57BL/6J male mice, c-fos
Introduction
A number of observations suggest that smoking and nicotine can regulate mood in both human subjects and animal models. In clinical studies, depressed subjects have an approximately 50% higher incidence of smoking than the general population (Diwan et al., 1998; Glassman et al., 1990; Kessler, 1995). In addition, nicotine patch can reduce symptoms of depression in non-smokers (Salin-Pascual et al., 1995) whereas smoking cessation can exacerbate symptoms of depression (Glassman et al., 1990). Animal studies have also shown that chronic nicotine administration can elicit antidepressant-like effects in rats both in the learned helplessness (Semba et al., 1998) and the forced swim (Djuric et al., 1999; Tizabi et al., 1999) paradigms. Taken together, these studies suggest that the nicotine in tobacco modulates neuronal systems regulating mood.
Nicotine exerts its effects by binding to, activating and desensitizing nicotinic acetylcholine receptors (nAChRs) in the central nervous system and autonomic ganglia (Picciotto, 1998). α4/β2-containing (α4/β2*) nAChRs combined with the α5, α6 or β3 subunits are the most widely expressed nAChRs in the central nervous system, and also have the highest affinity for nicotine, whereas α7* nAChRs form functional homomers and are highly expressed in the hippocampus and cortex, but also found in most other brain regions (Zoli et al., 1998). The observation that increased acetylcholine levels results in depression (Janowsky et al., 1972) whereas nicotine administration can decrease depressive symptoms (Salin-Pascual et al., 1995) appears paradoxical, however, chronic administration of nicotine (particularly as delivered through nicotine patch) can desensitize rather than activate nAChRs (Reitstetter et al., 1999), resulting in functional antagonism (for reviews see Gentry et al., 2002; Quick et al., 2002). This suggests that blockade rather than activation of nAChRs might have antidepressant effects. This hypothesis is supported by the fact that mecamylamine, a non-selective nAChR antagonist, decreased symptoms of depression in patients with Tourette’s syndrome (Dursun et al., 1999; Mihailescu et al., 2000; Salin-Pascual et al., 2003), and has antidepressant-like properties in mice (Caldarone et al, 2004; Rabenstein et al, 2006).
Studies in knockout mice have demonstrated that the absence of β2* nAChRs throughout development can lead to antidepressant-like phenotypes (Caldarone et al., 2004). Moreover, amitriptyline, a classical antidepressant, has no effect in these animals, strongly suggesting that β2* nAChRs are involved in the function of a classical antidepressant. This study also showed that subthreshold doses of the nicotinic antagonist mecamylamine and the tricyclic antidepressant amitriptyline could combine to result in antidepressant-like effects, supporting the idea that blockade of nAChRs might be antidepressant. We hypothesized that if blockade of nAChRs results in antidepressant-like behavior, interference with endogenous acetylcholine signaling though these nAChRs might also result in an antidepressant-like response. We therefore used cytisine, a partial agonist of β2* nAChRs and a full agonist at β4 nAChRs (Picciotto et al., 1995) in several tests of antidepressant efficacy. This is of particular interest since recent studies suggest that partial agonists of α4/β2* nAChRs may be useful in smoking cessation (Coe et al., 2005). Additionally, we furthered our behavioral analyses by testing locomotor activity and anxiety-like behaviors, because both could be major confounds in the different assessments we used in this study. Finally, we assessed c-fos expression, an immediate-early gene commonly considered to be a marker of neuronal activity, to identify neurobiological correlates of the antidepressant-like effects of cytisine.
Materials and Methods
Animals
Three-month-old C57BL/6J (B6) male mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were immediately grouped 4 per cage under standard conditions (temp. 21±2°C, 12:12 light-dark cycle, lights on at 7:00 a.m.). Food and water were available ad libitum. After a two-week rest period, mice were marked on their tail with a permanent marker for identification and were randomly assigned to one of the different treatment groups (saline, cytisine and mecamylamine; n = 8/group). Each animal received the same treatment throughout the different experiments. All procedures were approved by the Yale University Animal Care and Use Committee.
General testing conditions
Mice were habituated to the testing room at least 30 min before any behavioral evaluation. All tests took place between 12:00 a.m. and 5 p.m. The testing area was dimly lit to limit stress or anxiety (about 20 lux). For each behavioral assay, the experimenters were blind to treatment.
Treatment - Experimental design
Cytisine (0.2, 0.6, 1.0 and 1.5 mg/kg, i.p., Sigma Aldrich), and mecamylamine (1 mg/kg, i.p., Sigma Aldrich) were diluted in phosphate buffered saline (abbreviated “saline”, pH 7.1). Saline or drug was injected i.p. 30 min prior to the tail suspension test or the forced swim test. The two tests were separated by 48 hr. After the latter test, saline or drug was injected daily (between 9 and 11 a.m.) for 21 days and mice were subsequently tested in the novelty suppressed feeding test before their daily injection (n = 8/group).
A second group of animals was tested for locomotor activity after acute (30 min) or chronic (21 days) treatment with cytisine (1.5 mg/kg, i.p.) or mecamylamine (1 mg/kg, i.p.). 24 hr after the second locomotor activity test and 30 min after their daily injection of saline or drug, mice were tested in the light/dark box. 24 hr after the anxiety test, mice received their daily injection and were sacrificed 1 hr later. The brain was then removed and processed for immunohistochemistry (n = 8/group).
Behavioral assays
Tail suspension test
Mice were suspended by the tail with a paper clip attached with adhesive tape about 5 mm from the end of the tail. Time spent immobile was recorded over the 6 min test. After completion of the test, mice were returned to a holding cage until all cage-mates were tested. After completion of the experiment, all mice were returned to the home cage and transferred back to the holding room.
Forced swim test
Mice were placed in a beaker (18 cm in diameter) filled with 15 cm of water (~ 25 °C), with care taken not to put the nose of the mouse below water level. Time spent immobile during the 15-min testing period was recorded. The 15 min test was chosen based on our prior experience with C57BL/6J mice, since this strain shows very little immobility during the first 5 min of testing (Caldarone et al., 2004; Caldarone et al., 2003; Mineur et al., 2006b) the time point often used in other strains of mice and in rats. After testing, each mouse was placed in a heated holding cage (30–35°C) with bedding covered by a paper towel. After each mouse was tested, animals were returned to the holding room.
Novelty-suppressed feeding test
The protocol for novelty-suppressed feeding was based on previously published paradigms (for a review, see Dulawa et al., 2005). Mice were weighed and food was removed from their cage. Twenty four hr after removal of food, mice were transferred to the testing room, placed in a clean holding cage and allowed to habituate for at least 30 min. The testing apparatus consisted of a clear Plexiglas enclosure (40 × 40 × 17 cm), with a lid. The floor was covered with 2 cm of corncob bedding. A small piece of mouse chow was placed in the center of the arena on a piece of white circular filter paper (diameter: 9.5 cm). At the start of the experiment, each mouse was placed in the corner of the testing area, and the time to the first feeding event was recorded. Immediately after the mouse began to eat, the subject was removed and placed alone for 5 min in its original home cage with a pre-weighed piece of lab chow. At the end of the 5-min period, the amount of food consumed was determined. After all mice from a single cage were tested, mice were returned to their home cage.
Locomotor activity measurement
Mice were placed in a clean rat cage (48 × 22 × 18 cm) for 20 min. Locomotor activity was recorded using the Optomax system (Columbus instruments, Columbus, Ohio, USA). All mice from the same cage were tested at the same time in separated locomotor boxes. Subjects were returned to their home cage at the end of the test.
Light/Dark box
Anxiety was tested in the Light-Dark box as previously described (Guillot et al., 1996). Briefly, at the beginning of the test, each mouse was placed in an apparatus consisting of two opaque Plexiglas compartments of the same size (18 × 10 × 13 cm; light compartment illuminated by a 100 W desk lamp through a transparent Plexiglas cover). Animals cross from one compartment into the other through a small opening between them. Each mouse was placed in the illuminated compartment and observed for 5 min after the first entry into the dark compartment. A mouse whose four paws were in the other box was considered to have changed compartments. Behavioral variables analyzed were time spent in the light compartment (sec) and number of transitions between the light and dark compartments.
Immunohistochemistry
Mice were anesthetized by an overdose of chloral hydrate and were then quickly perfused intra-cardiacally with chilled PBS (0.1 M, pH 7.3) followed by chilled 4% paraformaldehyde (PFA) for about 10 min each (~100 ml of each solution per animal). Brains were subsequently removed from the skull and post-fixed for 24 hr in PFA at 4°C. After fixation, samples were placed in PBS (0.1 M, pH 7.3) with 30% sucrose for cryoprotection. Brains were then stored in sucrose at 4°C until slicing.
Forty-micron sections were cut with a microtome. Sections were labeled with a c-fos primary antibody (1:2000; Cell signaling, Danvers, MA 01923) at 4°C overnight, followed by a peroxidase-conjugated anti-rabbit secondary antibody (1:2000; Vectastain Elite, Vector laboratories Inc, Burlingame, CA, USA) for 1 hr at room temperature as free-floating sections in mesh wells. Staining was performed with reagents from a di-amino-benzadine (DAB) kit (Vector laboratories Inc, Burlingame, CA, USA). One of every 6 slices was then mounted onto gelatin-coated slides, covered with mounting media (Vectashield, Vector laboratories Inc, Burlingame, CA, USA), and sealed with a cover slip.
Photomicrographs of each region of interest were taken with a Nikon DIAPHOT inverted microscope at 200X and a digital camera attached to the microscope. c-fos positive cells were counted using NIH image software and macros based on those described in (Mineur et al., 2002b). In brief, gray-scale images were converted into a binary image (when a pixel was below a specified gray level, it appeared white; above the threshold, the pixel was black) and about 50 cells were randomly chosen across the different sections and assessed for their size and shape. A range of size (in pixels) was then derived from these observations, along with a general range of shape patterns. Subsequently, any cell with a size within the range previously established was automatically counted by the software. To test for reliability, this method was repeated twice, and showed >95 % replication between runs. The experimenter was blind to condition during the analyses.
Statistical analysis
Data were analyzed by analyses of variance (ANOVA) with Treatment as main factor, using Statview 5 (SAS institute, Cary, NC, USA). α was set at 5%.
Results
In the tail suspension test (Fig. 1A), mice injected with cytisine showed significantly less immobility at the 1.5 mg/kg dose compared to saline-treated animals (F1,14=13.15, p=0.003), as did mice injected with mecamylamine (1 mg/kg; F1,14=19.28, p=0.0006). Treatment with other cytisine doses showed a trend to induce decreased immobility but did not reach significance compared to saline treatment.
Figure 1.
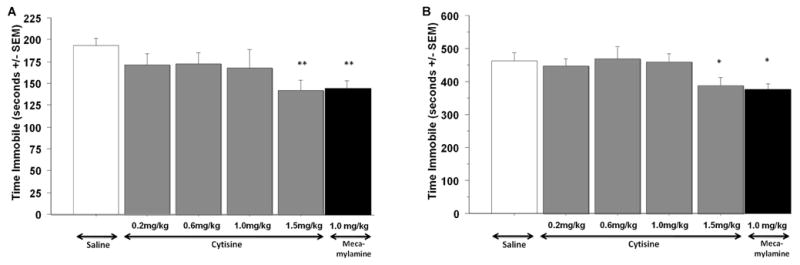
Total time spent immobile in the tail suspension (A) and in the forced swim (B) tests by C57BL/6J male mice treated with saline, cytisine and mecamylamine. Error bars represent SEM. * P< 0.05, ** P< 0.01. N=8 for each treatment group.
Similarly, in the forced swim test (Fig. 1B), mice treated with cytisine (1.5 mg/kg) spent significantly less time immobile than control animals treated with saline (F1,14=4.72, p=0.047), as was seen following mecamylamine treatment (F1,14=4.87, p=0.045). Again, the lower doses of cytisine (<1.5 mg/kg) did not show significant effects compared to saline treatment.
As has been reported for classical antidepressants previously, chronic (21 days, 1.5 mg/kg), but not acute (1.5 mg/kg), cytisine treatment decreased the latency to initiate feeding as compared to saline treatment (F1,13=15.63, p=0.0017 and F1,14=0.006, p=0.94, respectively; Fig. 2). A similar effect was observed in mecamylamine-treated animals (F1,13=7.16, p=0.019), but the mecamylamine effect was significantly different from the cytisine response (F1,14=8.75, p=0.0104). Weight loss after fasting or food intake after the test were not different across treatment groups (F2,20=1.26, p=0.30 and F(2,20)=0.206, p=0.82, respectively; data not shown).
Figure 2.
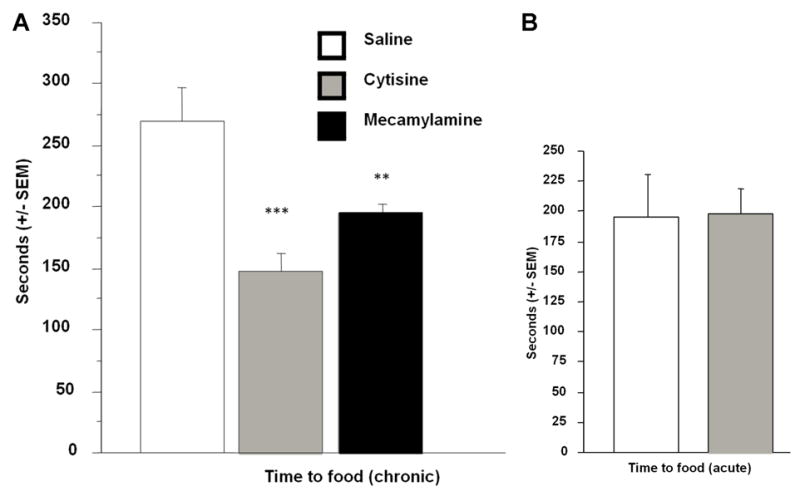
Latency to initiate a feeding episode in the novelty-suppressed feeding test in male C57BL/6 mice. Effects of acute cytisine treatment is represented on the right. Error bars represent SEM. ** P<0.01, *** P<0.001. N=8 for each treatment group.
In the light/dark test, cytisine treatment increased time spent in the dark compartment compared to saline treatment (F(1,13)=7.17, p=0.019; Fig. 3A). Locomotor activity in the box was unchanged by cytisine treatment (number of crossings: F(1,13)=1.56, p=0.23, data not shown). No change in overall locomotor activity was detected between treatment-groups either after acute (F(1,14)=0.62, p=0.44, Fig. 3B) or chronic cytisine treatment (F(1,14)=0.004, p=0.95, data not shown).
Figure 3.
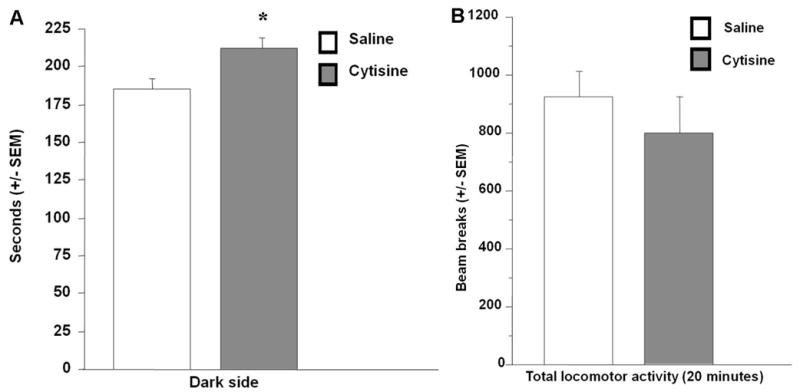
Total time spent in the dark compartment of the light/dark box test after acute cytisine or saline treatment in male C57BL/6 mice (A). Total number of beam breaks in an open-field for 20 minutes after acute injection of cytisine of cytisine or saline in male C57BL/6 mice (B). Error bars represent SEM. * P<0.05. N=8 for each treatment group.
An overall reduction of c-fos immunostaining was observed in mice treated with cytisine compared to the saline treated group (F(1,112)=3.45, p=0.03). A dramatic reduction of c-fos immunostaining was observed in the basolateral amygdala (Fig. 4) after cytisine treatment (F1,14=18.51, p=0.001), similar to what was observed with mecamylamine (F(1,14=26.20, p=0.0003).
Figure 4.
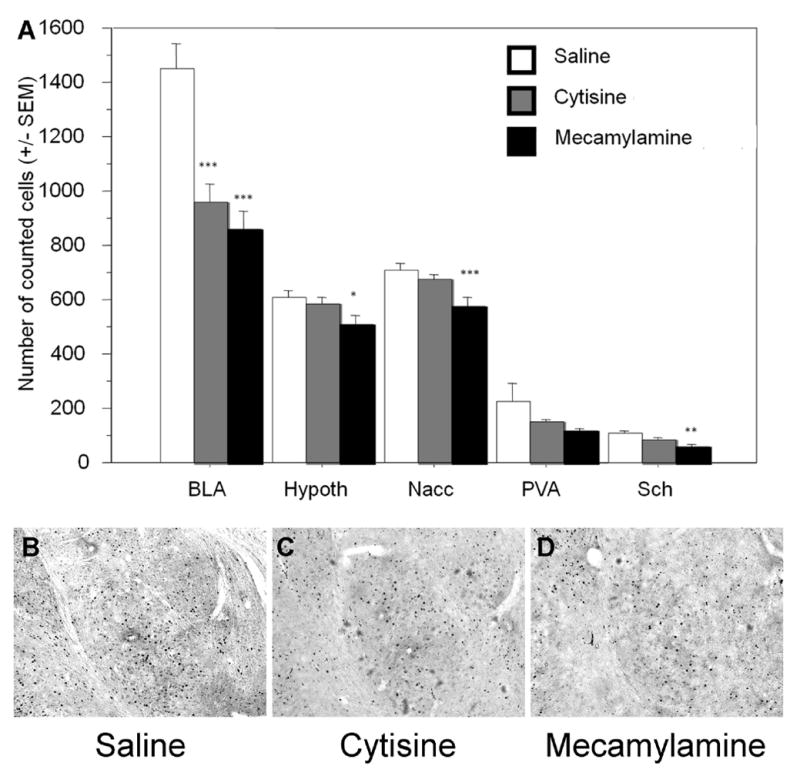
A. c-fos immunostaining in brain areas of C57BL/6J male mice, following 24-days of treatment with saline, cytisine or mecamylamine. BLA: basolateral amygdala; Hypoth: hypothalamus; Nacc: nucleus accumbens; PVA: paraventricular nucleus; Sch: suprachiasmatic nucleus. Errors bars represent SEM. * p<0.05; ** p<0.01; *** p<0.001. N=8 for each treatment group. B, C, D. Examples of c-fos immunostaining in the (baso)lateral amygdala in saline-, cytisine-, and mecamylamine-treated animals.
No other cytisine-induced differences in c-fos immunoreactivity were significant, although there was a general trend toward a reduction of c-fos staining in each brain region studied. However, mecamylamine showed a more pronounced effect than cytisine, with a significant reduction of c-fos staining in the hypothalamus (F1,14=5.48, p=0.03), the nucleus accumbens (F1,14=10.14, p=0.0072), and the suprachiasmatic nucleus (F1,14=11.09, p=0.005).
Discussion
The potential antidepressant-like effects of cytisine, a partial agonist of α4/β2* nAChRs and a full agonist at α3/β4* nAChRs, was investigated in several rodent models of antidepressant efficacy. Acute cytisine treatment had antidepressant-like effects in both the tail suspension and the forced swim tests. These effects did not appear connected to the full agonist properties of cytisine at α3/β4* receptors because similar results were obtained when animals were treated with the broad nAChR antagonist mecamylamine. These findings support the hypothesis that blockade of ACh signaling through α4/β2* nAChRs leads to antidepressant-like effects. These results are in agreement with studies showing mecamylamine has antidepressant-like properties in mouse models of antidepressant efficacy (Caldarone et al, 2004; Rabenstein et al, 2006) and that mice lacking the gene encoding the β2 subunit of the nAChR are unresponsive to the classical tricyclic antidepressant amitriptyline (Caldarone et al., 2004) and to the antidepressant-like effects of mecamylamine (Rabenstein et al, 2006).
The tail suspension and forced swim tests are responsive to acute injection of antidepressants, while antidepressants are only effective after chronic administration in depressed human subjects. Tail suspension and forced swim are also sensitive to acute injection of anxiolytic and psychotropic medications (for review see Cryan et al., 2005); Cryan et al., 2002; Cryan et al., 2004). We therefore also tested cytisine in the novelty-suppressed feeding test, a model of antidepressant efficacy known to respond to chronic antidepressant treatment and acute anxiolytic administration (Bodnoff et al., 1988; Dulawa et al., 2005). We observed an antidepressant-like effect of chronic, but not acute, cytisine administration in this paradigm, consistent with the effects of classical antidepressant medications, but not anxiolytic agents, in the novelty suppressed feeding test. The observation that mice did not differ significantly in weight, free-feeding or locomotion across treatment groups implies that motivation to obtain food, differences in metabolism or differences in activity were not responsible for the difference in latency observed in the novelty-suppressed feeding test.
Since anxiolytic agents can have effects in rodent depression models, we tested mice in the light/dark test of anxiety-like behavior following cytisine treatment. Subjects that received an injection of cytisine were somewhat more likely to stay in the dark compartment, compared to saline-treated mice, suggesting that if anything, cytisine-treated animals were more anxious. While unexpected, this strongly suggests that the effect we observed in the novelty-suppressed feeding test was not due to an anxiolytic effect of cytisine. These results are somewhat inconsistent with a recent study of cytisine in the elevated plus maze, another test of anxiety-like behavior (Carrasco et al., 2006). No significant differences were found between cytisine and saline groups in this study, but the doses used were lower than in the present study (0.35 and 0.175 mg/kg vs 1.5 mg/kg), which may account for the disparity. Additionally, the genetic background of the mice was different in the two studies (NMRI vs C57BL/6J) and genetic differences have been widely reported in anxiety-like behavior (Mineur et al., 2006a; Mineur et al., 2002a; Voikar et al., 2001).
It is not known which brain regions are important for the antidepressant-like effects of cytisine. We therefore used c-fos immunoreactivity as a measure of neuronal activation to determine whether specific patterns of neuronal activation or inhibition could be identified. Brains areas involved in dopaminergic and cholinergic neurotransmission did not show differences across treatment groups. For instance, there were no differences in c-fos immunoreactivity in the ventral tegmental area or the pedunculopontine tegmental area. However in the amygdala, a significant reduction of c-fos immunoreactivity (~50 %) was observed after chronic cytisine treatment, which was similar to the effect of mecamylamine. Several other brain regions showed a trend towards a reduction of c-fos expression after cytisine treatment, whereas the broad antagonist mecamylamine lead to a significant reduction of c-fos activity compared to saline. These data potentially implicate the BLA in both the acute and chronic actions of cytisine. Both the TST and FST respond to acute antidepressant treatments through unknown mechanisms, but it is possible that an initial change in c-fos levels could contribute to the acute behavioral response of cytisine in these tests. While we cannot rule out the involvement of other brain regions, these data suggest that blockade of nAChRs, and more particularly α4/β2* nAChRs, leads to a robust reduction of activity in the neurons of the basolateral amygdala, which may account for the antidepressant-like effects in this study.
The role of the amygdala in mood disorders including depression has long been established. Imaging studies in families affected by unipolar and bipolar depression have identified abnormal metabolism in the amygdala characterized by increased blood flow and glucose metabolism (Drevets, 2001). This could lead to excitotoxicity and neuronal death, and it has been suggested that hyperactivation may be responsible for the decrease in amygdala volume observed in the same patients (Drevets, 2001). Another recent study demonstrated that amygdalar perfusion was reduced following sleep deprivation-based antidepressant treatment (Clark et al., 2006). These observations are in accordance with studies showing a reduction of amygdala c-fos expression after chronic antidepressant treatment (Beck et al., 1995), whereas acute antidepressant administration can increase c-fos activation (Slattery et al., 2005). Thus, the decrease in c-fos immunoreactivity in the amygdala observed here following cytisine treatment is consistent with the idea that decreased activity in this brain area would be antidepressant-like.
Taken together, these data are the first to identify antidepressant-like properties of cytisine, and suggest that its ability to block α4/β2* nAChRs may be critical for this behavioral effect. Further, this study suggests that the antidepressant properties of cytisine may be mediated through a reduction of neuronal activity in the basolateral amygdala. These studies suggest that both antagonists and partial agonists of α4/β2* nAChRs would be interesting targets for the development of novel antidepressant drugs.
Acknowledgments
This work was supported by NIH grants DA13334/AA15632, MH77681, DA00436 and the NARSAD Foster Bam award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck CH, Fibiger HC. Chronic desipramine alters stress-induced behaviors and regional expression of the immediate early gene, c-fos. Pharmacol Biochem Behav. 1995;51:331–338. doi: 10.1016/0091-3057(94)00391-u. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Karthigeyan K, Harrist A, Hunsberger JG, Wittmack E, King SL, Jatlow P, Picciotto MR. Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology (Berl) 2003;170:94–101. doi: 10.1007/s00213-003-1518-7. [DOI] [PubMed] [Google Scholar]
- Carrasco MC, Vicens P, Vidal J, Redolat R. Effects of co-administration of bupropion and nicotinic agonists on the elevated plus-maze test in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:455–462. doi: 10.1016/j.pnpbp.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Archibald SL, Fennema-Notestine C, Braun DR, Thomas LS, Sutherland AN, Gillin JC. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Res. 2006;146:43–51. doi: 10.1016/j.pscychresns.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;13:13. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW. Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res. 1998;33:113–118. doi: 10.1016/s0920-9964(98)00045-0. [DOI] [PubMed] [Google Scholar]
- Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav. 1999;67:533–537. doi: 10.1016/s0031-9384(99)00091-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Kutcher S. Smoking, nicotine and psychiatric disorders: evidence for therapeutic role, controversies and implications for future research. Med Hypotheses. 1999;52:101–109. doi: 10.1054/mehy.1997.0623. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. Jama. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Guillot PV, Chapouthier G. Intermale aggression and dark/light preference in ten inbred mouse strains. Behav Brain Res. 1996;77:211–213. doi: 10.1016/0166-4328(95)00163-8. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Kessler DA. Nicotine addiction in young people. N Engl J Med. 1995;333:186–189. doi: 10.1056/NEJM199507203330311. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Drucker-Colin R. Nicotine, brain nicotinic receptors, and neuropsychiatric disorders. Arch Med Res. 2000;31:131–144. doi: 10.1016/s0188-4409(99)00087-9. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Belzung B, Crusio WE. Effects of unpredictable chronic mild stress on anxiety-like behavior and despair in mice. Behav Brain Res. 2006a doi: 10.1016/j.bbr.2006.07.029. In press. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Crusio WE. Behavioral and neuroanatomical characterization of FVB/N inbred mice. Brain Res Bull. 2002a;57:41–47. doi: 10.1016/s0361-9230(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR, Sanacora G. Antidepressant-Like Effects of Ceftriaxone in Male C57BL/6J Mice. Biol Psychiatry. 2006b doi: 10.1016/j.biopsych.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002b;12:39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Common aspects of the action of nicotine and other drugs of abuse. Drug Alcohol Depend. 1998;51:165–172. doi: 10.1016/s0376-8716(98)00074-x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, LeNovere N, Vincent P, Pich EM, Brulet P, Changeux JP. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not β2- or α7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology. 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Reitstetter R, Lukas RJ, Gruener R. Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther. 1999;289:656–660. [PubMed] [Google Scholar]
- Salin-Pascual RJ, Alcocer-Castillejos NV, Alejo-Galarza G. Nicotine dependence and psychiatric disorders. Rev Invest Clin. 2003;55:677–693. [PubMed] [Google Scholar]
- Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R. Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology (Berl) 1995;121:476–479. doi: 10.1007/BF02246496. [DOI] [PubMed] [Google Scholar]
- Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Morrow JA, Hudson AL, Hill DR, Nutt DJ, Henry B. Comparison of alterations in c-fos and Egr-1 (zif268) expression throughout the rat brain following acute administration of different classes of antidepressant compounds. Neuropsychopharmacology. 2005;30:1278–1287. doi: 10.1038/sj.npp.1300717. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Jr, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


