Abstract
Introduction:
Colonic pouches have been used for 20 years to provide reservoir function after reconstructive proctectomy for rectal cancer. More recently coloplasty has been advocated as an alternative to a colonic pouch. However there have been no long-term randomized, controlled trials to compare functional outcomes of coloplasty, colonic J-Pouch (JP), or a straight anastomosis (SA) after the treatment of low rectal cancer.
Aim:
To compare the complications, long-term functional outcome, and quality of life (QOL) of patients undergoing a coloplasty, JP, or an SA in reconstruction of the lower gastrointestinal tract after proctectomy for low rectal cancer.
Methods:
A multicenter study enrolled patients with low rectal cancer, who were randomized intraoperatively to coloplasty (CP-1) or SA if JP was not feasible, or JP or coloplasty (CP-2) if a JP was feasible. Patients were followed for 24 months with SF-36 surveys to evaluate the QOL. Bowel function was measured quantitatively and using Fecal Incontinence Severity Index (FISI). Urinary function and sexual function were also assessed.
Results:
Three hundred sixty-four patients were randomized. All patients were evaluated for complications and recurrence. Mean age was 60 ±12 years, 71% were male. Twenty-three (7.4%) died within 24 months of surgery. No significant difference was observed in the complications among the 4 groups. Two hundred ninety-seven of 364 were evaluated for functional outcome at 24 months. There was no difference in bowel function between the CP-1 and SA groups. JP patients had fewer bowel movements, less clustering, used fewer pads and had a lower FISI than the CP-2 group. Other parameters were not statistically different. QOL scores at 24 months were similar for each of the 4 groups.
Conclusions:
In patients undergoing a restorative resection for low rectal cancer, a colonic JP offers significant advantages in function over an SA or a coloplasty. In patients who cannot have a pouch, coloplasty seems not to improve the bowel function of patients over that with an SA.
The colonic reservoir has better bowel function than a straight anastomosis, but is not feasible in all patients. This study evaluates coloplasty in comparison with the J-pouch and straight anastomosis, and compares the functional outcome, quality of life, and complications.
The emphasis of surgery for low rectal cancers has undergone a distinct change from the oncologic importance of complete excision, adding a focus on a good functional result and the importance of maintaining quality of life (QOL). Since the late 1960s,1,2 when the colonal anastomosis were first described, surgeons have strived to balance a good oncological outcome with QOL. These efforts are exemplified by use of the circular stapler,3 definition of minimal safe margins,4–6 total mesorectal excision,7 and neoadjuvant therapy.
Lazorthes et al8 and Parc et al9 described the colonic J-pouch (JP) as an addition to the technique of coloanal anastomosis aimed at providing the reservoir function that is lacking in a straight coloanal anastomosis (SA). The pouch works by providing a segment of bowel with no functional peristalsis. Although functional outcome improved after a JP there existed evacuation difficulty10,11 that was resolved to some extent by defining the optimal length of a JP12 and level of anastomosis where a reservoir function has an advantage.13 Studies of patients with a colonic JP compared with an SA have shown that the antiperistaltic or reservoir capability of the colonic JP gave a better functional outcome in the short term.10,14,15 However urgency of defecation with the JP has not shown to be decreased by any study.3,12,14,16
Not all patients can have a JP incorporated into a coloanal anastomosis. Limitations to the construction of a JP include a narrow pelvis, a bulky mesentery or large appendices epiploicae, mucosectomy, insufficient colonic length, or extensive diverticular disease.17 In such patients, coloplasty has been suggested as an alternative.
Z'Graggen18 and his group first described the coloplasty pouch in a porcine model. This followed its introduction in clinical practice.19,20 The coloplasty pouch is a way of providing a reservoir without the bulk or the loss of length of a JP. Thus, it could potentially fit in a narrow pelvis or when the mesocolon was fat laden and bulky. The exit conduit from the coloplasty reservoir could fit/traverse the anal sphincters as far as an SA can. When the coloplasty pouch was compared with the JP in some randomized, controlled trials (RCT), and limited RCT, it showed results comparable to the JP21,22 and superior to the SA.23 However, no randomized trial has prospectively compared the functional outcome of all 3 procedures.
Other procedures described in the quest for achieving a good functional outcome have been the side to end coloanal anastomosis,24,25 which has been shown to have a similar outcome to the JP.
The purpose of this prospective RCT was to compare the functional outcome and QOL of patients who undergo a coloanal anastomosis with or without a reservoir for low rectal cancer and to study the complications that occur.
PATIENTS AND METHODS
A prospective randomized controlled multicenter trial was designed to enroll patients undergoing sphincter saving surgery for low rectal cancer. Six sites from the United States, 3 European, and 1 Australian site enrolled patients, after obtaining approval from their local institutional review board and ethics committee. The period of enrollment began in November 2000 and ended in June 2004.
The inclusion criteria were patients younger than 80 years, with a resectable low rectal cancer, where a coloanal anastomosis could be accomplished, which would lie within 0 to 4 cm of the dentate line. Exclusion criteria included metastatic disease, synchronous or metachronous colon cancer, previous colectomy, inflammatory bowel disease, pelvic radiation for other malignancies, and patients who were unable to complete or comprehend the preoperative questionnaire.
The enrollment was carried out by the surgeon or research personnel, and study-specific education was administered. After informed consent, patients were asked to fill out the preoperative questionnaires, which included the SF-36 QOL form, a urinary and bowel questionnaire, which included the American Society of Colon and Rectal Surgeon's fecal incontinence severity index (FISI), and a sexual function questionnaire, which was gender specific and used the Sexual Health Inventory for Men. Demographic details, preoperative assessment, intraoperative details, and complications were uniformly recorded on a datasheet that was sent to all the sites.
Randomization was carried out after oncologically acceptable resection, and confirmation of the feasibility of a coloanal anastomosis. A standard surgical technique was used, including total mesorectal excision, an intended minimal distal clearance margin of 2 cm and a loop ileostomy, which was intended to be closed within 3 months unless it was delayed because of unforeseen complications or adjuvant chemotherapy. The JP was standardized to a 5-cm length and the coloplasty pouch to 8 to 10 cm length with an exit limb that would measure 4 to 6 cm in length. Randomization was carried out by envelopes that were sequentially numbered and were prepared by the Department of Biostatistics and Epidemiology at the Cleveland Clinic Foundation, Cleveland, Ohio. These were sent to the participating centers in batches of 5 as patients were enrolled.
Patients were grouped after a low anterior resection was carried out, to either a JP eligible group or a JP ineligible group. The JP eligible group was one in which creation of a JP was technically feasible. These patients were randomized to receive either a JP or a coloplasty (CP-2). When it was established that a JP was not advisable in a specific patient, the patient was assigned to the JP ineligible group and randomized to receive either an SA or a coloplasty pouch (CP-1). The technique of the anastomosis (handsewn or stapled) was left to the discretion of the individual surgeon. Measurements of the level of anastomosis from the dentate line and the length of the JP and coloplasty pouch were taken intraoperatively. Other intraoperative details that were also recorded included tumor mobility, dissection plane with respect to the fascia of Denonvilliers, if any lysis of adhesions was carried out, other procedures that were performed, estimated blood loss, and use of antiadhesive products. The patients’ postoperative course was monitored, and the incidence and treatment of intraoperative and postoperative complications were recorded by the primary investigators at each site. Patients filled out questionnaires similar to those filled out at the preoperative screening visit at 4, 8, 12, and 24 months from the date of surgery. The only difference in the postoperative questionnaire was that the female sexual function questionnaire asked the same questions as those asked preoperatively, but asked for a comparison to the preoperative status for each question. Patients who had a delayed ileostomy closure had their forms marked as preileostomy closure. The data was collected from all sites and stored in a database created for this study at the Cleveland Clinic Foundation. Investigator meetings were held annually to make sure all sites kept to the standard set for data collection, adherence to the protocol, and setting quality checks for the data. Analyses were performed by the biostatistician using R version 2.3.1 (www.R-project.org).
Sample Size Calculation
Power and sample size calculations were performed during the design of the study for the bowel movement and SF-36 outcomes. Assumed standard deviations for bowel movement frequencies were approximated by the square root of mean values, as in the case of Poisson distributions. SF-36 scores were assumed to have a standard deviation no more than 10 points, as in the general population. It was estimated the study would be balanced between JP eligible and ineligible patients, and balanced between randomized groups within them. It was determined that a total sample size of N = 288 patients would then provide at least 80% power for comparisons of randomized groups with respect to mean bowel movements at α = 0.05 when true mean differences were 0.9 bowel movements or higher. Differences between randomized groups of at least 6 points in mean SF-36 scores would be detected with at least 95% power when compared at α = 0.05 with total N = 288. The study concluded with an imbalance of 70% JP eligible patients, resulting in less statistical power than anticipated within the JP ineligible cohort. As a result, observed group differences of similar magnitudes in the 2 cohorts may display differing levels of statistical significance. Therefore, comparisons between cohorts based on differing levels of significance are ill-advised.
Statistical Analysis
Categorical data were summarized overall and within randomized groups as frequencies and percentages, while quantitative variables were summarized by means and standard deviations, or by median with interquartile range (IQR) as deemed appropriate. Graphical displays of function parameters at individual time points display estimates of means and their confidence intervals. Treatment groups were compared with respect to quantitative data and ordinal categorical data (eg, categorized frequency of medication use) using Kruskal-Wallis tests, which in the case of 2-group comparisons are Wilcoxon rank sum tests. Comparisons with respect to nominal categorical data were performed with χ2 tests, or alternatively by Fisher exact test tests when low observed frequencies suggested possible inaccuracy of the χ2 test. P values from individual statistical tests are reported, and a level of α = 0.05 was used to define statistical significance for individual tests.
RESULTS
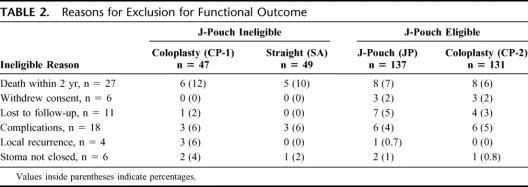
A total of 364 patients were enrolled and randomized. After excluding patients (Table 2), who were lost to follow up, withdrew consent, were ineligible because of complications and further surgery or had local recurrence and underwent an abdominoperineal resection, 297 patients were assessed for functional outcome and QOL. All 364 patients are included in the demographic and complications results.
TABLE 2. Reasons for Exclusion for Functional Outcome
The 4 randomized groups had 47 patients in the CP-1 group, 49 in the SA group, 137 in the JP group and 131 in the CP-2 group. The consort diagram (Fig. 1) illustrates the patient recruitment and follow-up.
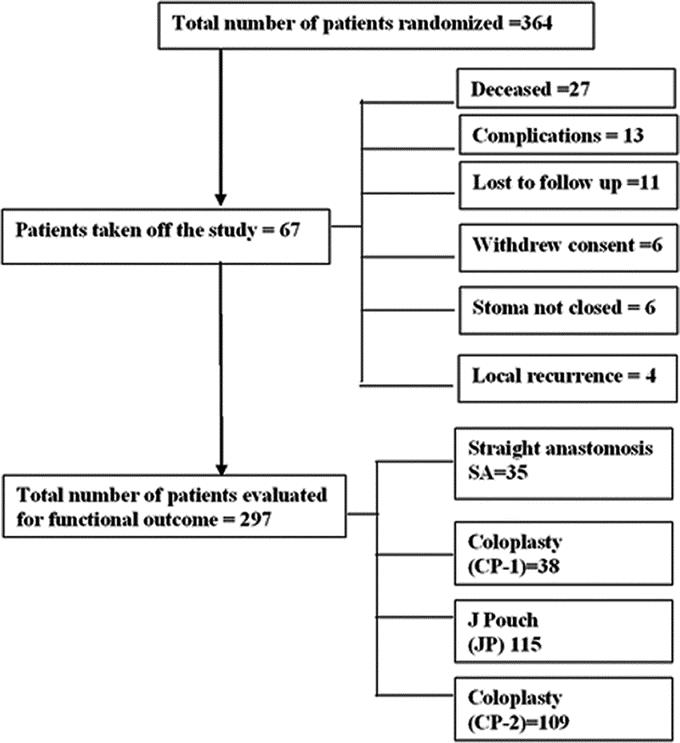
FIGURE 1. Consort diagram to show enrollment and randomization process.
Demographics
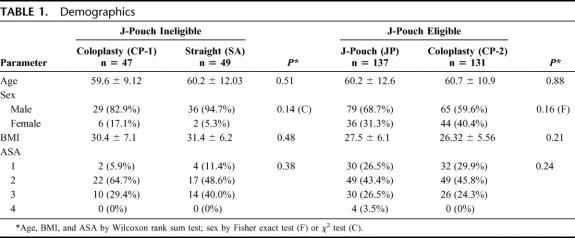
All groups were well matched for age at surgery, gender, duration of symptoms, body mass index (BMI) and associated comorbidities. However when we compared JP eligible with JP ineligible patients, ineligible group had a higher BMI (mean, 30.5 ± 6.3) than the eligible (mean, 26.9 ± 5.9) P = <0.001 (Wilcoxon rank sum test) (Table 1).
TABLE 1. Demographics
Twenty-seven patients (7.4%) died during the 2-year follow-up period. Of these, 1 was a perioperative death (0.3%) due to a massive cerebrovascular accident. Eleven patients died because of metastatic disease. Apart from the patients who died 45 patients were considered ineligible for functional assessment. Eighteen of these had surgery for complications, 4 had local recurrence and underwent an abdominoperineal resection, 6 patients did not have their stoma reversed for various reasons ranging from poor sphincter control to patient preference, 6 patients withdrew consent, and 11 were lost to follow-up (Table 2).
Presenting Symptoms and Preoperative Findings
The most common presenting symptom was rectal bleeding in 284 of 364 (78.0%) patients followed by changes in bowel habits in 133 (36.5%) patients. Abdominal pain and a significant weight loss were the next common symptoms seen in 31 (8.5%) and 29 patients (8.0%) patients. The rectal tumor was an incidental finding at endoscopy in 46 (12.6%) patients and on digital rectal examination in 9 (2.5%) patients. A rectal cancer causing symptoms of obstruction was seen in 4 (1.1%) patients. The median duration of symptoms before presentations for the entire cohort was 3 months (IQR 1–5).
A family history of colon cancer was reported by 60 of 342 (17.5%) patients with 49 (14.1%) claiming a first degree relative with colorectal cancer. Two hundred eighty-two of 342 (82.5%) patients reported no family history of colon cancer.
The tumor was located anteriorly in 93 (25.5%) patients, posteriorly in 94 (25.8%) patients, 47 (12.9%) in the right lateral quadrant, 76 (20.9%) in the left lateral quadrant and was circumferential in 62 (17.0%) patients. The median distance of the tumor from the dentate line was 3.5 cm (IQR 2–5.5).
Two hundred eleven patients (58.0%) underwent preoperative radiotherapy, of which 186 received (51.1%) received chemotherapy as well. One patient received only chemotherapy (0.3%).
Complications
Complications were strictly defined. Anastomotic separation was recorded when diagnosed clinically or radiologically diagnosed and if it caused a delay in the closure of the loop ileostomy. Anastomotic leak was defined radiologically and when treated with computed tomography-guided, transanal or open drainage. Bowel obstruction was recorded when it delayed primary hospital discharge or warranted a separate admission and conservative or surgical treatment was carried out.
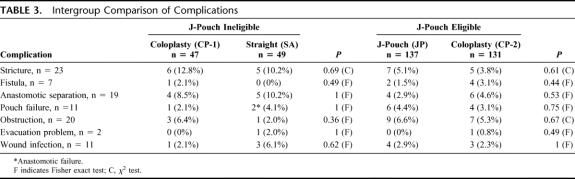
Overall 117 (32%) patients had complications that ranged from wound infection to pouch failure. Table 3 summarizes the complications within the various groups, and does not show a preponderance of any particular complication in any group. Five patients with unresolved strictures had additional surgery. Of these, 1 had a stricturoplasty and resolved subsequently, and another had a colostomy. Two patients had the pouch excised and had a permanent stoma. Two patients with fistulas had resolution of their symptoms, 2 were permanently diverted, 2 underwent repair and were subsequently healed, and 1 underwent a Soave procedure. Pouch ischemia accounted for 5 of 11 pouch failures and all cases were diverted. Two patients, 1 with a fistula and 1 with an anastomotic separation who lost their pouch subsequently underwent a lateral coloanal anastomosis. Another patient had a Soave procedure carried out when the pouch failed. Nine of 11 pouch failures were not salvageable. One patient who had an SA also was diverted.
TABLE 3. Intergroup Comparison of Complications
We studied associations between study variables and complications to identify potential predictors and found that the use of an antiadhesive agent was associated with a higher pouch failure rate (P = 0.03), and this was not noted with pelvic sepsis or anastomotic separation.
Functional Outcome
Two hundred ninety-seven patients were evaluated for functional outcome.
Bowel Function
Bowel function was reported as bowel movements (daily, day, and night), pad usage, urgency, clustering, continence scores, and usage of antidiarrheals and medication for constipation.
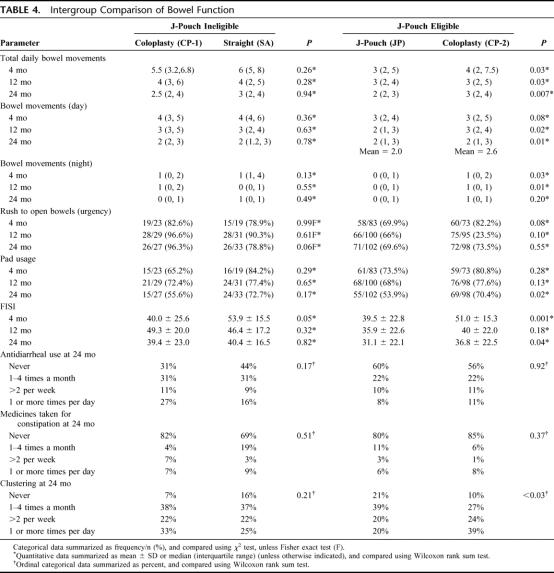
All 4 groups showed a decline in the number of daily bowel movements at 12 and 24 months when we compared their function to baseline (Fig. 2). However, for the total number of daily bowel movements (Table 4), the JP group had a fewer than CP-2 at 4 months (P = 0.03), 12 months (P = 0.03) and at 24 months (P = 0.007). Differences were also seen with night bowel movements and bowel movements during the day.
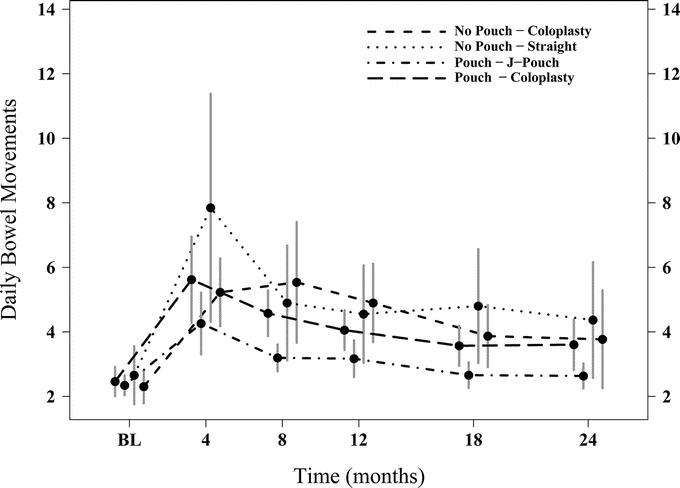
FIGURE 2. Graphic representation of total daily bowel movements of all 4 groups over 24 months. Mean and 95% CI at each time point.
TABLE 4. Intergroup Comparison of Bowel Function
Urgency, reported as “rush to open bowels” (Table 4), was not significantly different between the 4 groups although at 24 months the SA group was lower than the CP-1 group (79% vs. 96%, P = 0.06). Clustering (Table 4) at 12 and 24 months was more often seen in the CP-2 group than the JP group at 12 (P = 0.01) and 24 (P = 0.03) months, but was not significantly different in the SA and CP-1 group at any time point. At 24 months fewer patients in the JP group used pads (P = 0.01) while the SA and CP-1 groups were not significantly different (Table 4). The CP-1 and SA groups showed a trend to difference in pad usage at 4 months (56% vs. 73%, P = 0.17) similar in magnitude to the difference between CP-2 and JP groups (54% vs. 70%, P = 0.02). The statistical significance for the latter comparison, while not for the former, could be a matter of difference in sample sizes.
The use of antidiarrheals (Table 4) in any group any time point was not significantly different as was the use of medication for constipation. The FISI recorded at the various time points (Fig. 3) showed significantly lower scores in the JP group at 4 (P = 0.001) and 24 (P = 0.04) months indicating higher continence than the CP-2 group (Table 4).
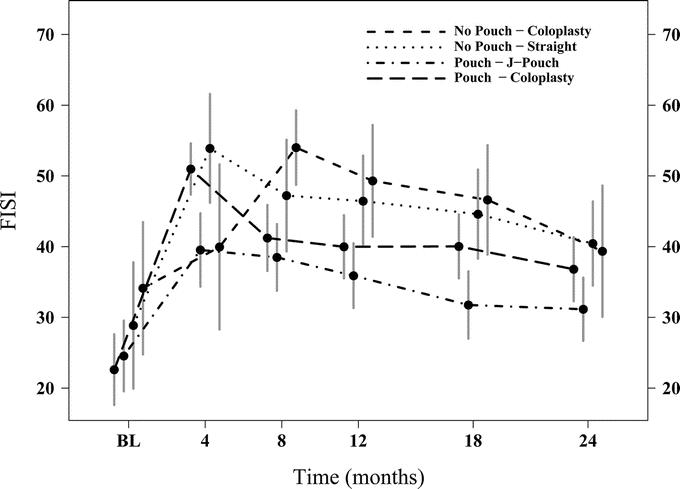
FIGURE 3. Graphic comparison of Fecal Incontinence Severity Index (FISI) scores over 24 months among all 4 groups. Mean and 95% CI at each time point.
Sexual Function
Sexual function in the men is reported as a total score of 5 questions each of which was scored from 0 to 5, for a total that ranged between 0 and 25. Higher scores reflected a better sexual function. Male sexual function declined from baseline and was the same across all 4 groups with low scores at 24 months. Change from preoperative scores was not different between the groups.
Female sexual function was assessed if the patient answered yes to the question that asked if they were sexually active. Female sexual function was the same between SA and CP-1 groups. However when the JP and CP-2 groups were compared at 12 (P = 0.04) and 24 (P = 0.01), months more women in the CP-2 group were sexually active.
Quality of Life
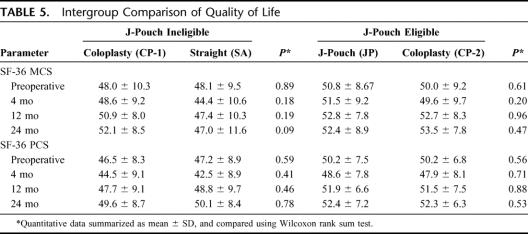
QOL was evaluated by measuring the Physical (PCS) and Mental component (MCS) scales from the SF-36 questionnaire. Although the scores of PCS and MCS did not attain any significance between the groups at any given time that they were evaluated, the CP-1 group had a higher MCS score than the SA group at 24 months. No difference was also noted when we compared the change in the scores from the preoperative values to 24 months (Table 5).
TABLE 5. Intergroup Comparison of Quality of Life
DISCUSSION
The purpose of constructing a colonic pouch is to reduce frequency and urgency of stool. This has been attributed to the reservoir capability of the pouch. Continence is maintained by the reflexes of the anal sphincter complex that are intact after a low anterior resection.26 Despite this there occurs a dysfunction of evacuation described as the “anterior resection syndrome”27 Hence, the quest for an ideal reservoir to replace the rectum is ongoing. Most prospective trials have compared the JP with the coloplasty pouch21,28,29 or the JP with the SA,13,15,30,31 others have retrospectively assessed all 3.32 These have demonstrated the superiority of the function of the JP over the SA and shown that the function of the JP and coloplasty pouch were comparable. Heriot et al33 concluded after their meta-analysis that the evidence suggested that JP was functionally superior to the SA, but that more trials were needed to make a conclusive statement about the coloplasty. This prospective randomized trial has compared the functional aspects of all 3 procedures after a low anterior resection. Our study has taken note of the previous studies which have shown the superiority of the JP over the SA, and hence, we divided the patients into 2 major groups, those in whom a JP is feasible and those in whom it is not. Therefore, the randomization process did not directly compare the patients who received a JP with those who received an SA. The patients who had a JP had better function than those who received a coloplasty, and this was not only in the number of bowel movements but also in the FISI and clustering. Fürst et al28,31 have postulated that the functional capacity of the JP is superior not because of the reservoir capability but because of its reduced propulsive motility. Koninger et al34 have found that the reservoir capacity of a coloplasty pouch was comparable to that of a JP and that urgency and incontinence were more frequent in patients with a limited pelvic floor motility. However, this was not seen in this trial. Historically, the JP was shown to have evacuatory problems, which resolve with a smaller pouch.12 In this series, a minority of patients in every group took laxatives, and daily bowel movements were significantly fewer in patients with a JP and fewer patients in this group reported clustering. The functionally superior results of the coloplasty pouch over the SA documented by various authors were not seen in this study, and this may have been due to the small sample size of the 2 groups or as to the study design, which restricted this comparison to a subgroup of patients ineligible for a JP, with higher BMI, narrow pelvis, and a more difficult rectal dissection.
There has been no consensus on whether the reservoir capability of the JP confers a long-term functional advantage with studies showing that it does10,15,35 and does not14 offer benefit beyond 24 months. In this study, the function of the JP did not decline over 24 months and was superior to that of the coloplasty pouch or the SA and conclusively proves that contrary to conventional wisdom the reservoir capability of the JP does not decline over time.
QOL measures however show that the QOL at 24 months is similar in all 4 groups. In our patients, QOL seems to be independent of differences in bowel function, which may reflect the relative insensitivity of the SF-36. Perhaps a disease-specific instrument would have been more reflective of differences in function. There was no significance difference in complications between any of the groups, showing that each of the surgical options is safe. The absolute complications were similar to those seen in other studies.36–38 The low perioperative mortality 0.3% speaks of the caliber of the participating surgeons and postoperative care.
CONCLUSIONS
This trial has shown that the JP is functionally superior to the coloplasty pouch and is recommended for all patients in whom a coloanal anastomosis is required where the pouch is practicable. The coloplasty pouch did not produce better function than the SA in our study, possibly because that arm of the study was underpowered. It remains an option however, as there is some evidence suggesting improved short-term function.
The long-term effects of the JP continue to be studied on this cohort of patients. Other alternatives like the side to end anastomosis, which is technically easier to perform than the JP or coloplasty pouch should be studied in larger multicenter, randomized trials to show their short- and long-term benefit.
ACKNOWLEDGMENTS
The authors thank Dr. J. C. Church for his invaluable help in editing the manuscript, the Department of Colorectal Surgery at Duke University, Durham, North Carolina, for also submitting patients for the trial, and all the research coordinators at all the centers who helped in data acquisition specifically Ann Dugan and Jane Bast (Cleveland, OH), Sean O'Brien (Burlington, VT), Kari McKee (Atlanta, GA), Joyce Turk (Pittsburgh, PA), Anna Williford and Wendy Kaiser (Louisville, KY), Ms. Margit Bauer (Regensburg, Germany), and the Délégation Interrégionale à la recherche clinique d'lle France Hôspital St. Louis, Paris.
Discussions
Dr. Robert D. Fry (Philadelphia, Pennsylvania): I must admit some disappointments in the results. I believe that, in large part due to pioneering efforts of many of the participants of this trial, especially Dr. Fazio, most surgeons would agree that a colonic J-pouch provides a superior functional result to a straight coloanal anastomosis. I had hoped that this study would demonstrate that a coloplasty is at least equivalent in function to a J-pouch, and Dr. Fazio's previous studies indicated that that should be expected. Alas, such are the treacheries of randomized and controlled trials.
Still, I wonder if this study completely sounds the death knell for the coloplasty. The authors have volunteered that the unexpectedly small size of the group that was ineligible for the J-pouch may have been too small of a sample size, or perhaps there were problems in this group with a higher BMI, narrow pelvis and more difficult dissection that negated the anticipated superiority of the coloplasty.
Fifty-eight percent of the patients received preoperative radiation therapy; 51% also received chemotherapy. I couldn't tell if the effect, or lack thereof, of radiation therapy was evaluated for this paper. Is it possible that patients that did not receive preoperative radiation therapy had a better functional result than those that did, and could the study be stratified to consider the influence of neoadjuvant therapy in these separate groups?
Along the same line, did the stage of the cancer have any effect upon functional outcome? I would assume the more advanced cancers were more likely to be treated with preoperative chemoradiation, and I would further assume that the operation in these patients would be more technically difficult. Were a significant number of patients considered ineligible for a J-pouch because of the technical difficulties associated with advanced cancer or with neoadjuvant therapy?
Finally, could you comment on the anatomy of the proximal component of the anastomosis? Did this consist of descending colon in all patients, or did some patients have the proximal component, be it J-pouch, coloplasty, or unaltered bowel, comprised of the sigmoid colon? I would think there could be expected to be a significant difference of function if the coloplasty were performed using sigmoid colon rather than descending colon, and I couldn't tell from this presentation if the sigmoid colon were used as a proximal component of the anastomosis.
Dr. Massarat Zutshi (Cleveland, Ohio): We concur about the disappointment in the final analysis. Based on our previous studies, we too had anticipated that the function of the 2 colonic pouches would be the same. But data are what they are, and even data from the individual centers showed us the same kind of result.
Regarding the small numbers of ineligible patients for the J-Pouch, our previous studies have shown that about one-third of the patients who undergo surgery for lower rectal cancers, especially males would be ineligible for the J-pouch. And in this study we had 97 of the 364 patients, which amounts to about 27% of the patients, who were ineligible. Our sample size was based on the hypothesis that there would be a difference between the function in patients undergoing a coloplasty and a straight anastomosis.
Dr. Fry, you rightly pointed out that the body mass index was higher in the pouch ineligible group. But that higher BMI did not favor the straight anastomosis, as it was high for both the straight anastomosis as well as the coloplasty. We also did not find adequacy of colon length to be a problem in randomization or performing an anastomosis to the anus.
On the question of chemoradiation, we do agree that the chemoradiation does affect the function after a coloanal anastomosis. However, in this study, in each group there were between 55% and 65% of patients who underwent chemoradiation, which would affect function almost equally in all the groups.
Regarding the technical difficulties, we carried out the randomization after the resection. And we addressed this problem of randomization in every investigator meeting and we did not find any difficulty in the randomization process.
Lastly, about what part of the colon was used for anastomosis, the protocol did not state which part of the colon was to be used. However, it was the stated practice of our unit to use the descending colon routinely, and we did not record these data for the study.
Dr. Fabrizio Michelassi (New York, New York): You conclude that the J-pouch was superior to coloplasty or straight coloanal anastomosis. And if one looks at pad usage, a commonly used surrogate for frequent incontinence, at 24 months it is not really different among the 4 groups. Indeed, of 297 patients, at 24 months you had 196 in whom it could be evaluated. Of these, 151 used it, almost 75%. This seems to me a very high percentage of patients using protective pads at 24 months. I would like to know whether you have an explanation for that.
Were the majority of these anastomoses handsewn rather than stapled? How many patients received preoperative radiation therapy versus postoperation therapy? And to a certain extent in consideration of this study as international, was there a difference in pad usage in the 4 different nations that participated in this study?
A second question, I wonder whether you could give us an idea of the percentage of dehiscence at the coloplasty site in the pouch ineligible patients versus the pouch eligible patients. One would surmise that patients ineligible for the J-pouch had a left colon that reached with difficulty down to the pelvic floor, and therefore the coloplasty performed in these patients could be at a higher tension, could have higher tension than the coloplasty performed in patients that were eligible for a J-pouch.
Dr. Victor W. Fazio (Cleveland, Ohio): First of all, the reason for doing this kind of study is because of the known fact that the J-Pouch is superior to straight anastomosis. And yet there are cases, in my personal series a third of patients, who are J-Pouch ineligible because of a bulky mesocolon that is brought down through the narrow aperture of the anal sphincter mechanism, and the sometimes narrow pelvis in particular. This is especially so for a handsewn anastomosis where one is doing either an intersphincteric or, ultra low colorectal anastomosis/coloanal anastomosis.
Adequate reach is usually not the issue, at least from the studies that we have done in our section of this particular report as well as from what we gathered from the twice-a-year investigator reviews in-house with the other study group members. Rather, the difficulty with making a J-Pouch anal anastomosis was based upon local factors of the anorectal area and the narrowness of the pelvis rather than intestinal length.
Dr. Massarat Zutshi (Cleveland, Ohio): In regards to the question of pad usage, first, at 2 years there is a general agreement among those who use colonic reservoirs that there is a time limit of 1 to 2 years beyond which the differences in function between a straight anastomosis and a J pouch are similar due to the development of a “neoreservoir” function of the straight anastomosis. We asked the question whether the pad usage at 24 months was for continence (necessity) or just for the patient's peace of mind. In this study 13% of patients used it for necessity, 50% for peace of mind and 37% for both. We are not sure whether pad usage at 24 months was really related to continence and would not be a good surrogate for incontinence. In the study, patients from Germany and France have a little bit higher pad usage at 4 months than the patients in the US sites. At 24 months, the pad usage rate in patients from the US sites was the highest, and very close to the 4-month percentage, where as the percentages of pad usage in patients at the European sites declined. A majority of the anastomoses were stapled. Thirty-three percent of the patients received a handsewn anastomosis.
Regarding the question of radiation, 58% of the patients underwent preoperative radiation. And radiation did have an effect on the functional outcome of all these patients. There were less than 5 patients who received postoperative radiotherapy.
If defining dehiscence as either anastomotic separation or pelvic sepsis, there were 17 (9.6%) patients with dehiscence out of 178 coloplasty patients evaluated for complications. Among 131 pouch eligible coloplasty patients, 11 (8.4%) had dehiscence, while 6 (12.8%) of 47 pouch ineligible coloplasty patients had dehiscence. So there is a slight trend as you hypothesized, though not statistically significant.
Dr. Merril T. Dayton (Buffalo, New York): Was any consideration given in the study design to formally assessing sphincter function ala anorectal manometry? My concern is that if you acknowledge the potential impact of radiation treatment on sphincter function, if you are assessing incontinence, how can you be sure it is a function of the pouch design and not radiation weakened sphincters? And while there may have been an equal distribution of radiation patients in all groups, because of biological variability you cannot be sure that the effect of the radiation is evenly distributed. So, I would be interested in your thoughts on assessing anorectal strength in this group of patients.
Dr. Victor W. Fazio (Cleveland, Ohio): In our unit we assess sphincter function preoperatively and postoperatively, and measure compliance of the reservoir or nonreservoir. However, we did not mandate that in the study groups because many of the units just did not do this on a routine basis. But I think that all of the units would try to assess patients to use a sphincter-saving operation according to their established guidelines of clinical impression of sphincter adequacy.
With respect to the ultimate outcome, one's impression is that the compliance was greater in those patients who had reservoirs and that the anal sphincter function can certainly deteriorate as a result of the preoperative chemoradiation therapy. But since there was parity between all groups in the percentage of patients in whom chemoradiation was used, this nullified that concern. However, we do recommend that patients be assessed with anal physiology preoperatively.
Dr. Stanley M. Goldberg (Minneapolis, Minnesota): My question is this. In your introduction, you alluded to the end-to-side anastomosis. This is commonly referred to as the Joel Baker anastomosis. Have you given any thought to doing this type of anastomosis? It is a simpler anastomosis, there is 1 less suture line, and I think it gets around all the problems related to adding a reservoir to the low anastomosis. What are your thoughts about the Joel Baker anastomosis for reconstruction of the rectum in this situation?
Dr. Victor W. Fazio (Cleveland, Ohio): It is quite true that the side-to-end Baker anastomosis has experienced a resurgence of some popularity based on 2 studies.24,25
These reports would, on the face of it, appear to be of value. And one of them, I think, was a randomized trial with small numbers and short follow-up. This will require a much bigger study, probably multi-institutional, because it took a long time to do this current study with established techniques for performing a coloanal anastomosis.
Footnotes
Supported by all the centers except in France where it was funded by Délégation interrégionale àla recherche clinique d'lle France Hôspital St. Louis, Paris.
Reprints: Massarat Zutshi, MD, A-30 Cleveland Clinic Foundation, 9500 Euclid Ave., Cleveland, OH 44195. E-mail: zutshim@ccf.org.
REFERENCES
- 1.Turnbull R Jr, Cuthbertson A. Abdominorectal pull-through resection for cancer and for Hirschsprung's disease. Delayed posterior colorectal anastomosis. Cleve Clin Q. 1961;28:109–115. [DOI] [PubMed] [Google Scholar]
- 2.Parks A. Transanal technique in low rectal anastomosis. Proc R Soc Med. 1972;65:975–976. [PMC free article] [PubMed] [Google Scholar]
- 3.Goligher JC. Extended low anterior resection with stapled colorectal or coloanal anastomosis. Ann Chir Gynaecol. 1986;75:82–88. [PubMed] [Google Scholar]
- 4.Karanjia ND, Schache DJ, North WR, et al. Close shave in anterior resection. Br J Surg. 1990;77:510–512. [DOI] [PubMed] [Google Scholar]
- 5.Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg. 1983;198:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimeter rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patient's survival. Br J Surg. 1983;70:150–154. [DOI] [PubMed] [Google Scholar]
- 7.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. [DOI] [PubMed] [Google Scholar]
- 8.Lazorthes F, Fages P, Chiotasso P, et al. Resection of the rectum with construction of a colonic reservoir and coloanal anastomosis for carcinoma of the rectum. Br J Surg. 1986;73:136–138. [DOI] [PubMed] [Google Scholar]
- 9.Parc R, Tiret E, Frileux P, et al. Resection with coloanal anal anastomosis with colonic reservoir for rectal carcinoma. Br J Surg. 1986;73:139–141. [DOI] [PubMed] [Google Scholar]
- 10.Dehni N, Tiret E, Singland JD, et al. Long-term functional outcome after low anterior resection: comparison of low colorectal anastomosis and colonic J-pouch-anal anastomosis. Dis Colon Rectum. 1998;41:817–822, discussion 822–823. [DOI] [PubMed]
- 11.Hida J, Yasutomi M, Maruyama T, et al. Enlargement of colonic pouch after proctectomy and coloanal anastomosis: potential cause for evacuation difficulty. Dis Colon Rectum. 1999;42:1181–1188. [DOI] [PubMed] [Google Scholar]
- 12.Lazorthes F, Gamagami R, Chiotasso P, et al. Prospective, randomized study comparing clinical results between small and large colonic J-pouch following coloanal anastomosis. Dis Colon Rectum. 1997;40:1409–1413. [DOI] [PubMed] [Google Scholar]
- 13.Hida J, Yasutomi M, Fujimoto K, et al. Functional outcome after low anterior resection with low anastomosis for rectal cancer using the colonic J-pouch. Prospective randomized study for determination of optimum pouch size. Dis Colon Rectum. 1996;39:986–991. [DOI] [PubMed] [Google Scholar]
- 14.Joo JS, Latulippe JF, Alabaz O, et al. Long-term functional evaluation of straight coloanal anastomosis and colonic J-pouch: is the functional superiority of colonic J-pouch sustained? Dis Colon Rectum. 1998;41:740–746. [DOI] [PubMed] [Google Scholar]
- 15.Lazorthes F, Chiotasso P, Gamagami RA, et al. Late clinical outcome in a randomized prospective comparison of colonic J pouch and straight coloanal anastomosis. Br J Surg. 1997;84:1449–1451. [PubMed] [Google Scholar]
- 16.Dehni N, Schlegel D, Tiret E, et al. Effects of aging on the functional outcome of coloanal anastomosis with colonic J-pouch. Am J Surg. 1998;175:209–212. [DOI] [PubMed] [Google Scholar]
- 17.Harris GJ, Lavery IJ, Fazio VW. Reasons for failure to construct the colonic J-pouch. What can be done to improve the size of the neorectal reservoir should it occur? Dis Colon Rectum. 2002;45:1304–1308. [DOI] [PubMed] [Google Scholar]
- 18.Z'Graggen K, Maurer CA, Mettler D, et al. A novel colon pouch and its comparison with a straight coloanal and colon J-pouch–anal anastomosis: preliminary results in pigs. Surgery. 1999;125:105–112. [PubMed] [Google Scholar]
- 19.Z'Graggen K, Maurer CA, Buchler MW. Transverse coloplasty pouch. A novel neorectal reservoir. Dig Surg. 1999;16:363–366. [DOI] [PubMed] [Google Scholar]
- 20.Fazio VW, Mantyh CR, Hull TL. Colonic “coloplasty”: novel technique to enhance low colorectal or coloanal anastomosis. Dis Colon Rectum. 2000;43:1448–1450. [DOI] [PubMed] [Google Scholar]
- 21.Ho YH, Brown S, Heah SM, et al. Comparison of J-pouch and coloplasty pouch for low rectal cancers: a randomized, controlled trial investigating functional results and comparative anastomotic leak rates. Ann Surg. 2002;236:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pimentel JM, Duarte A, Gregario C, et al. Transverse coloplasty pouch and colonic J pouch - a comparative study. Colorectal Dis. 2003;5:465–470. [DOI] [PubMed] [Google Scholar]
- 23.Mantyh CR, Hull TL, Fazio VW. Coloplasty in low colorectal anastomosis: manometric and functional comparison with straight and colonic J-pouch anastomosis. Dis Colon Rectum. 2001;44:37–42. [DOI] [PubMed] [Google Scholar]
- 24.Machado M, Nygren J, Goldman S, Ljungqvist O. Similar outcome after colonic pouch and side-to-end anastomosis in low anterior resection for rectal cancer. Ann Surg. 2003;238:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber FT, Herter B, Siewert JR. Colonic pouches vs side to end anastomosis in low anterior resection. Dis Colon Rectum. 1999;42:896–902. [DOI] [PubMed] [Google Scholar]
- 26.Lane RH, Parks AG. Function of the anal sphincters following coloanal anastomosis. Br J Surg. 1977;64:506–509. [DOI] [PubMed] [Google Scholar]
- 27.Williamson ME, Lewis WG, Finan PJ, et al. Recovery of physiologic and clinical function after low anterior resection of the rectum for carcinoma: myth or reality? Dis Colon Rectum. 1995;38:411–418. [DOI] [PubMed] [Google Scholar]
- 28.Furst A, Suttner S, Agha A, et al. Colonic J pouch vs coloplasty following resection of distal rectal cancer. Early results of a prospective randomized pilot study. Dis Colon Rectum. 2003;46:1161–1166. [DOI] [PubMed] [Google Scholar]
- 29.Sailer M, Debus ES, Fuchs KH, et al. Comparison of different J-pouches vs. straight and side-to-end coloanal anastomoses: experimental study in pigs. Dis Colon Rectum. 1999;42:590–595. [DOI] [PubMed] [Google Scholar]
- 30.Hallbook O, Sjodahl R. Comparison between the colonic J pouch-anal anastomosis and healthy rectum: clinical and physiological function. Br J Surg. 1997;84:1437–1441. [PubMed] [Google Scholar]
- 31.Furst A, Burghofer K, Hutzel L, et al. Neorectal reservoir is not the functional principle of the colonic J-Pouch: the volume of a short colonic J Pouch does not differ from a straight coloanal anastomosis. Dis Colon Rectum. 2002;45:660–667. [DOI] [PubMed] [Google Scholar]
- 32.Remzi FH, Fazio VW, Gorgun E, et al. Quality of life, functional outcome and complications of coloplasty pouch after low anterior resection. Dis Colon Rectum. 2005;48:735–743. [DOI] [PubMed] [Google Scholar]
- 33.Heriot AG, Tekkis PP, Constantinides V, et al. Meta-analysis of colonic reservoirs versus straight coloanal anastomosis after anterior resection. Br J Surg. 2006;93:19–32. [DOI] [PubMed] [Google Scholar]
- 34.Koninger JS, Butters M, Rediecke J, et al. Transverse coloplasty pouch after total mesorectal excision: functional assessment of evacuation. Dis Colon Rectum. 2004;47:1586–1593. [DOI] [PubMed] [Google Scholar]
- 35.Ho YH, Seow-Choen F. Prospective randomized controlled study of clinical function and anorectal physiology after low anterior resection: comparison of straight and colonic J Pouch anastomosis. Br J Surg. 1996;83:978–980. [DOI] [PubMed] [Google Scholar]
- 36.Rullier E, Laurent C, Zerbib F, et al. [Conservative treatment of adenocarcinomas of the anorectal junction by preoperative radiotherapy and intersphincteral resection]. Ann Chir. 2000;125:618–624. [DOI] [PubMed] [Google Scholar]
- 37.Pollard CW, Nivatvongs S, Rojanasakul A, et al. Carcinoma of the rectum. Profiles of intraoperative and early postoperative complications. Dis Colon Rectum. 1994;37:866–874. [DOI] [PubMed] [Google Scholar]
- 38.Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;336:896–902. [DOI] [PubMed] [Google Scholar]