Abstract
Objective:
To test the hypothesis that comparably injured women, especially those in the hormonally active age groups, would manifest a better preserved hemodynamic response and tissue perfusion after major trauma than do men.
Summary Background Data:
The notion that premenopausal women are more resistant than men to shock and trauma has been shown in numerous preclinical models. However, human studies on the effects of gender on outcome after shock-trauma are less clear, and none has examined the effect of gender on the immediate postinjury response to major trauma.
Methods:
Prospective series of all patients at a Level I trauma center from January 2000 to December 2005. Study patients were all patients arriving to the trauma area of the emergency department and having a serum lactate drawn within 30 minutes of arrival. Demographic data, injury severity indices, blood utilization, and lactate levels were recorded. Lactate was used as a marker of the hemodynamic response to injury, because it has been shown to be an excellent and accurate indicator of inadequate tissue perfusion.
Results:
A total of 5192 patients were eligible for the study of which 4106 fulfilled the study requirements and were enrolled. Initial serum lactate levels were significantly lower in premenopausal (age 14–44) and perimenopausal (age 45–54) women than in men of the same age groups (P < 0.001), even though the Injury Severity Score of the women was significantly higher than that of the men (24 vs. 18; P < 0.1). When patients were stratified into major injury groups as well as groups receiving blood transfusions, the premenopausal women were also found to have lower initial serum lactate levels and receive less blood, while having a greater magnitude of injury as reflected in their Injury Severity Score.
Conclusion:
The data firmly establishes a proof of principle that hormonally active human women have a better physiologic response to similar degrees of shock and trauma than do their male counterparts. These gender-based differences should be taken into account in designing studies evaluating the response to shock-trauma.
A prospective clinical series of 4106 patients was carried out testing the hypothesis that women would manifest a better preserved initial hemodynamic response and tissue perfusion after trauma than do men. Based on lactate levels and transfusion requirements, this hypothesis was validated in premenopausal women although the magnitude of injury was greater in women than in men.
Emerging data describes a sexual dimorphism in the response to major injury or sepsis with differences demonstrated in immune function as well as susceptibility to organ injury and infection.1,2 These differences have been attributed to sex hormones, with the female sex hormone estradiol conferring protection and the male sex hormone testosterone increasing susceptibility to injury.1,2 Although some epidemiologic studies suggest that women survive sepsis better than men,3 the notion that gender might influence the clinical outcome after trauma or sepsis was largely ignored until experimental studies from Dr. Chaudry's laboratory documented that sex hormones modulate the immune response to shock and sepsis as well as improve survival.2 Since then, multiple clinical studies have seemed evaluating the influence of gender on survival, the development of organ failure, or infection after major trauma.4–14 Although the results of these epidemiologic trauma studies vary, with some showing gender-based benefits and others showing no differences between the sexes, certain general trends seem to exist. First, there seems to be a trend toward increased survival for younger (premenopausal) women which seems to be present only in women with more severe injuries.7,9,12 Additionally, several of the studies indicate that the incidence of posttraumatic infection and the multiple organ dysfunction syndrome (MODS) is less in the younger premenopausal female population suggesting that the reduced incidence of MODS may, at least in part, contribute to the lower mortality rate observed.
In light of emerging clinical studies and the work of Chaudry, we began experimental studies testing the basic hypothesis that female rats would be more resistant to acute trauma-hemorrhagic shock-induced gut and lung injury than would male rats.1 These gender studies confirmed that it is not only being female that is protective, but that the protection is related to the hormonal status of the rat at the time of injury. That is, the resistance to trauma-hemorrhage-induced organ injury varies over the estrus cycle with maximal protection during the proestrus and estrus stages of the cycle when estrogen levels are highest.15 Conversely, rats at the diestrus stage of the cycle, when sex hormones are lowest, demonstrate a degree of gut and lung injury that nearly approaches that of male rats.3 Thus, experimentally, there seems to be a clear, graded response to trauma-hemorrhage-induced organ injury that is related to the hormonal status of the rat at the time of injury. Given this rodent data, we believe that the results of the human studies become a little easier to interpret. Firstly, because the benefit of being female seems to exist primarily in the reproductive years, studies examining gender dimorphism to injury need to segregate hormonally active female patients as the potential cohort most likely to have a beneficial outcome. Additionally, it is important to recognize that hormonal status is severely altered following critical injury and illness.16,17 For example, Woolf et al have shown that estradiol levels in hormonally active women decreased markedly after injury,18 with postinjury estrogen levels approaching those seen in control postmenopausal women. This observation that female sex hormones decrease markedly over the first several days after a major injury would suggest that the maximal benefit to being female would primarily exist during the initial injury period. Consequently, we believed that studies looking at the effect of gender on outcome focusing on the immediate postinjury period would be most likely to identify sex hormone and gender-related differences in outcome. Thus, in the current study, we examined gender dimorphism in the early response to injury, as reflected by plasma lactate levels obtained within 30 minutes of hospital arrival as well as the need for early postinjury blood transfusions. Serum lactate levels were chosen as a marker of the hemodynamic response to injury, because serum lactate has been shown to be an excellent marker of inadequate tissue perfusion as well as a prognostic factor.19,20
PATIENTS AND METHODS
Study Design
On the basis of our gender-based rodent studies,1,3 we hypothesized that premenopausal women would manifest less of an initial physiologically deleterious hemodynamic response to trauma and shock than would male trauma patients, as reflected in plasma lactate levels obtained shortly after trauma. We also hypothesized that a relative resistance to the adverse hemodynamic consequences of trauma and shock would be manifest as a lesser need for blood transfusions in the premenopausal women than in the men when matched for the magnitude of the injury. Thus, the 2 primary outcome variables of the study were the initial lactate levels and blood transfusions. The study was not intended nor powered to evaluate survival differences between the groups, although this data was retrieved from our trauma registry data bank. The study was approved by the Institutional Review Board of UMDNJ—New Jersey Medical School.
From January 2000 to December 2005 serum lactate levels were drawn within 30 minutes of arrival to the trauma area of the emergency department on all trauma patients believed to require admission to our Level I trauma center. Initial data collected included demographics, body mass index (BMI), arrival Glasgow Coma Score, vital signs, and lactate levels, and subsequent data collected included blood products administered as well as injury severity score and maximum AIS severity by region plus hospital disposition. Exclusion criteria consisted of patients admitted with drowning, hanging or isolated spinal cord injuries as well as burn/inhalation injuries in the absence of major trauma. As shown in Figure 1, of the 16,617 patients who were seen in the trauma admitting area of the emergency department, 5192 were eligible for inclusion in the study. Of these patients 1086 were excluded due to failure to obtain a serum lactate level or the lactate level was drawn >30 minutes after arrival. Thus, 4106 patients were enrolled.
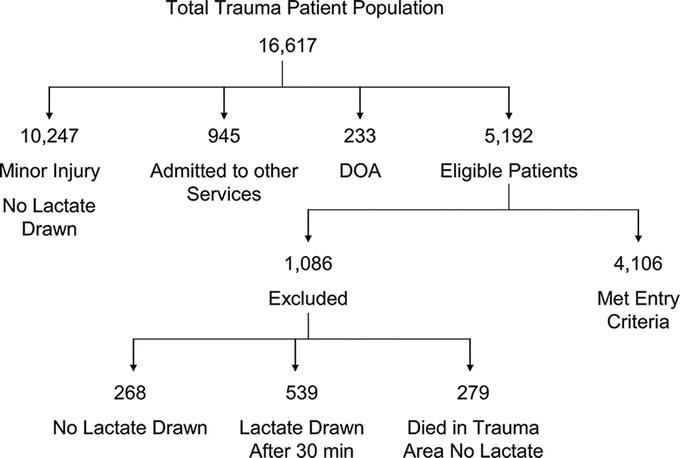
FIGURE 1. Summary of patient population and enrollment.
To further evaluate the lactate response a subgroup of patients with major injuries were evaluated. Patients were defined as having major injuries if they met the following criteria: (1) presence of major abdominal injuries with AIS of 4 or 5 or ≥2 AIS 3 injuries or penetrating injury with major blood loss, (2) ≥2 long bone fractures, complex pelvic fracture, flail chest and/or major vascular injury, (3) ISS ≥26 or (4) 3 or more injuries in a single body region with an AIS ≥3. This major injury subgroup consisted of 880 patients and represented approximately 21% of the 4106 enrolled patients.
Because older patients make less lactate19 and evidence less of a base deficit21 following comparable injuries when compared with younger individuals and that sex hormonal status varies based on age, we examined the data with respect to the following age groups: prepubescent (age <14 years), hormonally active or premenopausal (ages 14–44 years), perimenopausal in which hormonal status may be unclear (ages 45–54 years), and postmenopausal (age ≥55 years).
Statistics
Statistical tests were done using Analyze-it (version 1.68, Analyze-It Software, Leeds, England) and SPSS (version 14.0, SPSS, Chicago, IL). Univariate analysis was performed on the data with means (±sd) or percent reported. Ninety-five percent confidence intervals (95% CI) are given for continuous data for multivariate comparisons. Bivariate and multivariate analyses included contingency statistics (χ2, Kruskal-Walis) for categorical or nominal data and Student t test or analysis of variance (ANOVA) for continuous data. Actual significance values are reported. Appropriate diagnoses were done on the data and suitable transformations were done when necessary.
The first step in the analysis involved exploring the potential confounding effect of body mass (using BMI) on lactate production. A General Linear Model (GLM) analysis of covariance (ANCOVA) was done using lactate as the dependent, age and gender as the independents, and BMI as the covariate. The next steps involved running GLM ANOVA models for age and gender on initial lactate first for the entire data set and then for patients with major injury. This was followed by an exploration of lactate clearance in the first 24 hours (n = 462), employing a repeated measures ANOVA using sex and the age groups defined above. Provided an overall significant effect was found in the ANOVA as derived, Tukey's significant difference test was used for multiple comparisons with P < 0.05 being considered significant.
RESULTS
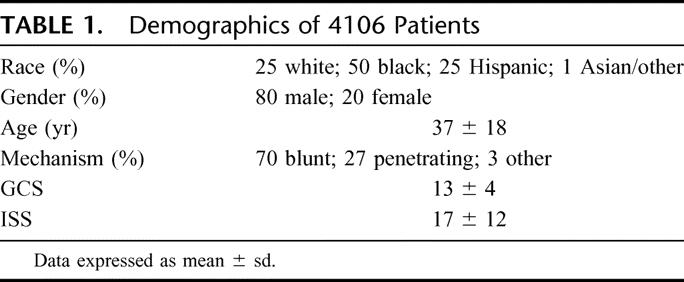
During this time period, there were 16,617 trauma activations generating 5192 eligible patients of whom 4106 were enrolled into the study (Fig. 1). The demographics of the 4106 patients are shown in Table 1. As expected most of the patients were men (80%) and blunt trauma (70%) was the most common mechanism of injury. The enrolled patients had a relatively high ISS (17 ± 12) which is reflected in 1285 of the 4106 patients (31%) going from the admitting area directly to the operating room. The total mortality rate was 10% (n = 403) with 57 patients dying in the trauma admitting area of the emergency department.
TABLE 1. Demographics of 4106 Patients
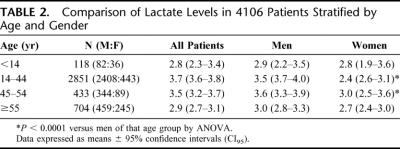
The mean and 95% confidence limits (CI) of the lactate levels for the entire cohort were significantly different between men (3.7 meq/L; CI95 3.62–3.85) and women (2.8 meq/L; CI95 2.67–2.99) (P < 0.001). When stratified by age, the differences in lactate levels between the men and women were only observed in the premenopausal age and perimenopausal age groups (Table 2). Thus, there was no difference in initial lactate levels in patients younger than 14 or older than 55 years. Although the initial lactate levels were higher in the men, one concern was that the larger overall body size and muscle mass of the men, irrespective of injury, could result in higher lactate levels. To account for this potentially confounding variable, heights and weights were recorded on all patients and BMI was calculated. The results of the ANCOVA found no significant effect for BMI on lactate (F = 0.75; P = 0.39). Thus, it is unlikely that size and muscle mass alone account for the gender-related differences observed. Furthermore, the magnitude of injury, as reflected by the ISS (mean ± sd), was greater in the premenopausal women (24 ± 13) than in men (18 ± 13) of the same age (P < 0.01). Another indication of hemodynamic stability is the need for blood transfusions. Although a similar percentage female versus male patients required blood transfusion in the first 24 hours after trauma (17% vs. 16%) as well as during their hospital course (31% vs. 27%), the women received fewer units of blood than did the men at both time points. This was true even though the transfused female patient subgroup had a greater degree of injury as reflected by a higher ISS (Fig. 2).
TABLE 2. Comparison of Lactate Levels in 4106 Patients Stratified by Age and Gender
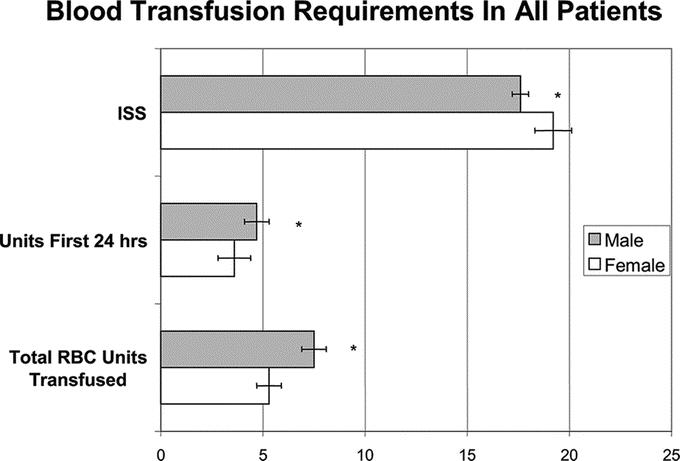
FIGURE 2. Women received less blood both during the initial 24 hour postinjury period as well as during their hospital course even though they had a higher ISS. *P < 0.01.
Because lactate clearance is an important prognostic factor,22 we next examined whether there was a gender difference in lactate clearance over the first 24-hour hospital period in the 460 patients who had serial plasma lactate levels measured. As can be seen in Figure 3, although the men had higher initial lactate levels than did women, the men rapidly cleared their lactate and within 8 to 16 hours the levels were comparable between the genders.
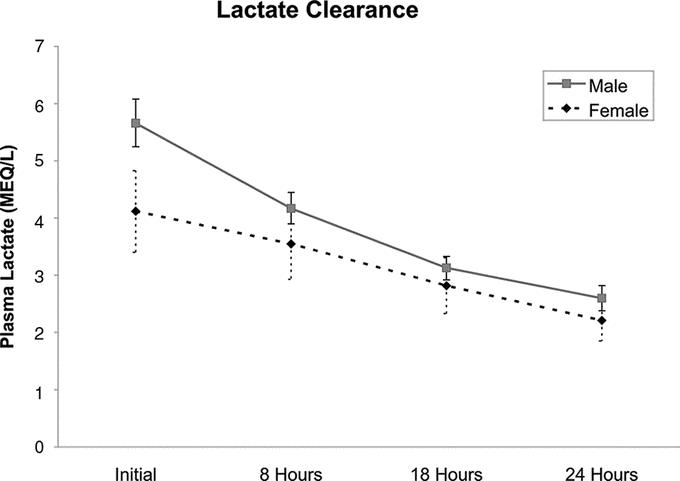
FIGURE 3. Twenty-four lactate clearance in 462 patients showing that males clear their lactates such that their levels become comparable to women. Data expressed as mean with 95% confidence intervals.
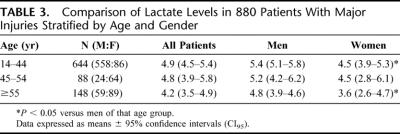
Because differences in the incidence of shock or the severity of injury between the gender groups could also account for lactate differences, we further analyzed the data for patients with major injuries. The major injury group included 880 patients older than 14 years and comprised 21% of the enrolled study group. Of these patients, 73% were male and 27% were female. In this major injury group, men also had higher initial lactate levels (mean 5.3 meq/L; CI95 5.0–6.6) versus women (mean 4.2 meq/L; CI95 3.6–4.7) (P < 0.001). When this patient subpopulation was stratified by age and gender, initial lactate levels were lower in the women than in the men in 14 to 44 age group (Table 3) even though the ISS of the women in this group was higher than that of the men (33 ± 13 vs. 30 ± 13; P = 0.02). The total number of blood transfusions was also less in the premenopausal women when compared with men (Fig. 4) even though the percentage of women receiving blood transfusions in the first 24 hours after trauma was similar to their male cohort population (31% vs. 30%) as was the total number of patients transfused (66% vs. 63%). Although the initial lactate levels were lower in the premenopausal patients with major injuries and they received less blood, the ISS of the transfused women was higher than that of the men (Fig. 4). However, there was no difference in mortality between the male (17.6%) and female (18.6%) patients.
TABLE 3. Comparison of Lactate Levels in 880 Patients With Major Injuries Stratified by Age and Gender
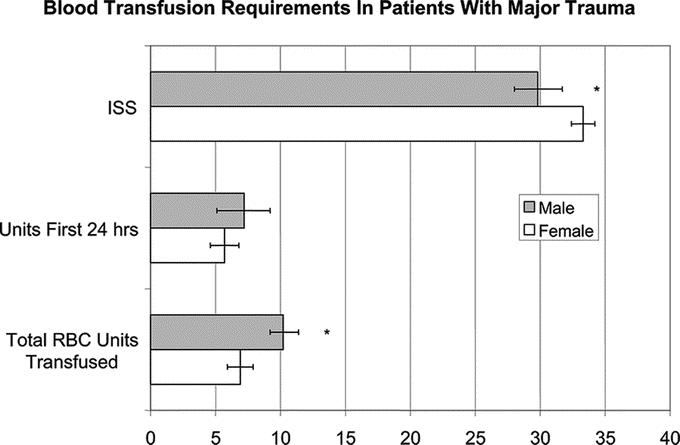
FIGURE 4. Women sustaining major trauma received less blood both during the initial 24 hour postinjury period (P = 0.24) as well as during their hospital course even though they had a higher ISS. *P < 0.01.
DISCUSSION
The major observation of this study is that gender has a significant effect on the response to trauma with women in the premenopausal and perimenopausal age groups having a reduced initial lactate response to their injury when compared with their male counterparts. This gender difference in lactate levels was not related to injury severity, BMI, or degree of shock. The decision to use initial lactate levels as an indicator of the body's response to injury and shock is based on the physiologic concept that increased lactate levels early after injury and shock states reflect tissue hypoxia and is a better marker of the adequacy of tissue oxygen delivery and hemodynamic status than is the blood pressure.23 In fact, elevated lactate levels and the rate at which they resolve have been shown to have strong prognostic values in the initial evaluation of both trauma and sepsis patients.19,22,24 This association between elevations in serum lactate levels and outcome indicate that a major component of the initial insult in shock and trauma is a failure of the circulatory system to deliver adequate oxygen to the tissues. In this context, lactate levels reflect lactate production, because lactate produced in cells diffuses passively across the cell membrane into the blood along a concentration gradient. Thus, the lesser degree of lactate production in the pre- and perimenopausal women after trauma indicates that these female patients are better able to maintain hemodynamic homeostasis and deliver oxygen to their tissues than are men. As such, these results support the general concept that women of these age groups adapt better to the initial and early physiologic stresses of trauma and shock than do men. This notion was further supported by the reduced need for blood transfusions in the female patients.
By prospectively assessing the initial response to trauma, this study differs from other epidemiologic studies investigating the question of whether a gender dimorphism exits in response to trauma between men and women. With the exception of the study by McKinley et al,25 the other trauma studies have focused on later events in the hospital course of these patients.4–14 Although in the aggregate these trauma studies support the general thesis that women have improved outcomes compared with men, they do have some limitations. For example, one limitation of many of these studies is that they have not segregated patients by age and hence potential hormonal status thereby resulting in the mixing of premenopausal, perimenopausal, and postmenopausal patients.5,8,11,14 Nonetheless, several studies that did stratify patients by age and injury severity did find a gender-based survival advantage for premenopausal women with ISS >15,7,9,12 and others documented a reduced incidence of sepsis and MODS.4,5,9,10,11,13,14 Although our study was not designed or powered to assess survival, the observation that pre- and perimenopausal women manifest less tissue hypoperfusion after trauma than do men could help explain the improved outcomes reported in the literature.
Recognizing that women, especially hormonally active premenopausal women, have improved outcomes, an important issue becomes the ability to translate this epidemiologic information into clinically possible therapeutic approaches. The accomplishment of this goal requires an understanding of the mechanisms by which sex hormones modulate the response to shock and trauma. Because sex hormone levels have been shown to rapidly change in both male and female patients after injury, sepsis, or stress,17,18,26–28 we believe that their modulatory effects are maximal at the time of injury and decrease over time. For example, testosterone levels have been documented to decrease by about 50% within 24 hours in critically ill men18,28 as well as in men with severe sepsis, septic or circulatory shock,17,26 while estrogen levels are increasing in these critically ill men.17,26 In contrast to men, there is little data on the effect of injury, stress, or sepsis on sex hormone levels in women. However, there is some data indicating that estrogen levels increase in postmenopausal women with severe sepsis, septic or circulatory shock just as observed in men.17 Thus, although human data is limited, it seems that sex hormones rapidly change in stressed and septic patients with testosterone decreasing in men and estrogens increasing in men and postmenopausal women. Consistent with this notion is a study of a mixed group of septic patients with a mean age of 55 years, in which sepsis-induced changes in both testosterone and estrogens occurred such that by day 7 the levels of these sex hormones were similar between the 2 genders.29 Based on this data, it is tempting to speculate that the observed decreases in testosterone and increases in estrogens reflect a physiologic adaptive response to severe stress or sepsis. Testing this notion will require further studies in which sex hormone levels are initially and serially measured and correlated with outcome.
The concept that elevating estrogen levels and decreasing testosterone may be beneficial is supported by a large amount of preclinical animal data. For example, the greatest resistance of female animals to organ injury after trauma-hemorrhage is during the periods of the estrus cycle in which estradiol levels are the highest.15 Furthermore, animal studies of shock and sepsis have shown that the administration of estrogen or testosterone depletion and blockade is protective in men, while estrogen depletion or blockade is deleterious in women.2 Thus, preclinical animal models indicate that both estradiol and testosterone are important modulators of outcome and that their levels at the time of injury or sepsis are the key determinants of outcome. Our results showing that initial lactate levels are lower in pre and perimenopausal female trauma patients complement these animal studies. Additionally, the clinical study by McKinley et al25 showing that women sustaining major trauma had a better early cardiovascular and hemodynamic response to volume resuscitation than did comparably injured male patients also is consistent with the notion that the level of sex hormones at the time of injury is important. Our current results also fit with a large body of human nontrauma data showing that gender influences the cardiovascular system under conditions of stress.30
Insights into the potential mechanisms by which sex hormones might modulate the initial and early responses to trauma and shock come from animal studies. These animal studies document that proestrus female rats, in which estradiol levels are high, are largely protected from the deleterious effects of trauma-hemorrhage on cardiac and hepatic function31,32 as well as gut and lung injury.15,33 The relative resistance of female rats, as opposed to male rats, seems to involve both the protective effect of estradiol and the deleterious effects of testosterone. One potential explanation for the lesser incidence and magnitude of early organ injury and dysfunction in the female rats as opposed to male rats subjected to trauma-hemorrhage is that cardiac function and organ blood flow is better preserved in the female animals during the postshock and resuscitative period.34 Our unpublished animal studies show that female rats maintain cardiac output and organ blood flow better than do male rats even during the shock period and that this preservation of cardiac output and organ blood flow is associated with less of a vasoconstrictive response to trauma-hemorrhage. The experimental observations that the cardiovascular and hemodynamic responses differ between male and female animals are consistent with in vitro and in vivo studies investigating the vasoactive properties of estradiol and testosterone. For example, estradiol has been found to promote vasodilation through a number of mechanisms including upregulating the vasodilators cNOS and carbon monoxide30,35 as well as by opposing or inhibiting the actions of several vasoconstrictor molecules including α-adrenergic agents, thromboxene A2 and endothelin.36–38 In contrast, testosterone has been shown to have myocardial depressant activities32 as well as to promote vasoconstriction by increasing thromboxane A2 production39 and potentiating the renin-angiotensin response.40 Thus, it is likely that the vasoactive properties of estradiol and testosterone are involved in differentially modulating the hemodynamic and vasoactive responses that occur during the initial and early posttrauma period and could therefore potentially account for the gender differences in lactate levels observed in the current study.
When one considers the possibility that a lesser lactate response reflects better tissue perfusion and a better preserved hemodynamic state, then the question arises of how might this beneficial hemodynamic state be transduced into improved clinical outcomes. Because tissue ischemia is a profound inducer of an augmented immunoinflammatory response,41 one possibility is that by decreasing the magnitude of tissue ischemia, female sex hormones limit the extent of the posttrauma inflammatory response and preserve a more normal immunoinflammatory state. This possibility is supported by the observation that the magnitude of the trauma-hemorrhage-induced immunoinflammatory response is significantly greater in male animals than in female animals and that this response is modulated by sex hormones.42,43 Furthermore, having a more normal and less stimulated initial posttrauma immunoinflammatory response would reduce the likelihood of a second posttrauma insult inducing an uncontrolled immunoinflammatory response, the so-called 2-hit phenomenon.41 A second possibility is that by better preserving tissue and organ blood flow, especially to the gut, organ function would be better maintained. The prevention of splanchnic hypoperfusion and gut ischemia may be especially important, because rodent,1 porcine,44,45 and even primate46,47 studies document that early posttrauma-hemorrhage-induced organ injury and inflammation is mediated by proinflammatory and tissue-injurious factors released by the ischemic gut into the intestinal lymphatics.
In summary, the current study documenting a gender difference in the initial response to shock-trauma provides potential insights into the mechanisms by which sex hormones might influence the clinical outcome. Further studies investigating specific outcome variables as well as stratifying patients by initial and serial sex hormone levels as well as age and magnitude of injury will be necessary to fully determine the role of sex hormones as modulators of the incidence of complications and death after major trauma.
Discussions
Dr. B. David Hoyt (Burbank, California): The question of whether sexual dimorphism exists in the inflammatory response following trauma is accepted in animal models. Receptor mediated signal transduction through the estrogen receptor protects against post-injury organ failure and mortality. It has been shown that following hemorrhage, sepsis or other forms of trauma, this response is the same. Recent studies even suggest that following profound hemorrhage, its protective effect is accompanied by metabolic down regulation of ATP production, a form of suspended animation, if you will.
The problem has been in showing this in clinical studies. Several large analyses, including one of our own, have come to different conclusions as to whether this is a clinically relevant phenomenon. The NIH-sponsored Resuscitation Outcomes Consortium currently has a pre hospital trial under consideration.
The present study is well designed and attempts to control for the degree of injury and uses age as a surrogate for estrogen status. It finds that there is a significant but modest effect in lactic acidosis in patients who are presumed to be hormonally active. This is accompanied by a significant increase in red cell transfusion. We have no data on outcome analysis for multiple organ failure, but mortality was not different.
This really questions the importance of the lactate difference of 1.1 milli equivalents if I read the manuscript correctly in the group as whole and only 0.9 milli equivalents in the sickest group. This could simply be a sex difference in lactate metabolism unrelated to estrogen. This leads me to several questions.
First, do you think this difference is clinically significant? And if so, in what regard?
Second, is the transfusion difference seen in the females accompanied by an increased incidence of trauma? Since this was a prospective trial, did you collect data on the time point of each patient in their estrogen cycle? Could we be missing a more profound effect if you had a subgroup analysis that was definitely under the effects of estrogen?
Are you comfortable with the technique of inquiry matching? Was this adequate, to account for confounding effects between age groups for the more severely injured patients? The number of patients in some of these groups is really quite small.
And finally, Dr. Deitch, do you carry birth control pills in your Lexus in the event that you are severely injured?
Dr. Edwin A. Deitch (Newark, New Jersey): Thank you, Dr. Hoyt. Let me begin by lumping 2 of your questions together. And that is in the matching of the patient groups and the question of whether the differences are clinically significant.
The questions that we asked pre-study were related to lactate levels and need for transfusions. We did not plan for outcome studies and did not use clinical nurses to collect outcome data.
But like all good surgeons, we tortured and manipulated the data in our trauma registry. And in fact, if we do look at that data and compare the sickest patients, we do find differences. That is, the females have more ventilator free days, they tend to have significantly less organ failure and sepsis. There was no difference in mortality.
We, however, or at least I, decided not to put this data in the manuscript because I think it remains speculative. Trauma registries have their problems, and what I would like to do is go forward with a more focused study looking at better defined populations, obtain sex hormone levels, and know that what we write is really going to be correct and maybe not add something to the literature that may not be fully true.
Your other question about would I take birth control pills. I think trauma surgeons will probably save more lives if they keep their testicles and testosterone intact than if they were to chemically lower it.
Dr. Frederick A. Moore (Houston, Texas): Your data is fairly consistent with a study we published several years ago in which we resuscitated major torso trauma patients who arrived in shock with a computerized protocol. We showed that females responded much better than males to a series of interventions that were aimed to optimize cardiac output. Our typical patient arrived in the ICU about 6 hours after trauma center admission after undergoing a hemorrhage control intervention. These patients arrive in the ICU vasoconstricted, anemic and with low cardiac outputs. A good response is characterized by a brisk decrease in systemic vascular resistance with a corresponding increase in cardiac output to fairly modest volume loading and blood transfusion. Can you comment on how sex hormones might modulate that response?
The second question concerns acute traumatic coagulopathy. It is becoming increasingly apparent that more severely injured torso trauma patients bleed more because their coagulation system is activated by the associated tissue injury. Do you have any idea if there was a difference in the early INRs between the male and female patients?
Dr. Edwin A. Deitch (Newark, New Jersey): Let me answer your last question first. We do not have any data on coagulopathy. I am sure if I go back to the charts we can, in fact, look at INR and begin to answer that question, and it is something that we should do.
Your first question is to me a very important question. In doing the study, one of the most important things to understand is, if gender or sex or hormones do affect outcome, what is the mechanism? Sex hormones have been shown to have a number of different effects, and I believe that their cardiovascular effects are most important. So in terms of cardiac function and in terms of micro-vascular perfusion, it is very clear that estrogen tends to be a vasodilator and cardio-protectant. It does this through a number of different means such as an increasing vasodilator factors such as nitric oxide or carbon monoxide and opposing vasoconstrictors. On the other hand, testosterone is a myocardial depressant, and also appears to increase vasoconstrictive systems thereby making tissue perfusion worse in the stress phase. So our working mechanistic hypothesis is that estrogen protects because of its beneficial efforts on the vascular system and we will go back and forth between the patient and the animal laboratory to validate this notion.
Dr. Donald E. Fry (Chicago, Illinois): I think one of the issues we can lay to rest is that it is probably not the mitochondria that are responsible for this since they all came from women to begin with.
Secondly, you did present in the manuscript data that index to lactate to BMI, and so I think adipose tissue level of estradiol synthesis is clearly not an issue at all.
The early response reflects an enhancement or amplification of the acute hyper response to inflammation. That is to say, it would appear that the males experienced enhanced perhaps vasodilatation, an enhanced shock state, enhanced acute blood loss. And it raises the issue that has been raised and discussed by others as to whether there are different models, different mechanisms that modulate or dampen our acute inflammatory response. Kevin Tracy has talked about inflammatory reflux. So what you are raising now are issues as to whether there are hormonal modulators.
Although I think this begs the question, is it estradiol or estrogens at all? Are there other hormonal differences that can be identified between the pre-menopausal female and the male that may address it? For example, is there a difference in 17 hydroxy steroid released under issues of stress between men and women that may potentially address the differences and that this may not be sex-linked hormones at all.
Finally, for a difference to be significant, it has to have significance relative to the outcome. And the data that you have presented really does not show a difference in the phenotype of the proposed genotypic differences that you are hypothesizing.
You have indicated that there were no organ failure differences. Were there any differences even in the incidence of infection? Were there white count differences? Were there early fever response differences between the male and the female? C-reactive protein would be an interesting indicator of acute inflammatory response.
It is very interesting data, but the question of its clinical relevance I still believe remains to be answered.
Dr. Edwin A. Deitch (Newark, New Jersey): In answer to your question, is there clinical relevance? As I mentioned to Dr. Hoyt, if we do sufficient data manipulation, we could have shown outcome differences in this paper using multiple subgroup analysis, I thought that this type of post analysis would be unfair, since the study was not designed to address these outcomes.
Dr. Charles E. Lucas (Detroit, Michigan): When we looked at a group of over 600 patients who received an average of 14 transfusions and a minimum of 8 units during the first 8 hours, we identified that female gender had a negative effect on outcome, namely survival. We assumed that this was due to the fact that a number of blood transfusions to an average size 55 kilo woman versus 70 kilo man were relatively greater in the woman.
When we looked at another group of patients who had severe blunt chest injury with multiple rib fractures, we also identified that female gender was associated with a higher injury severity score and a worse outcome. And we assumed that this was due to the fact that equal force on a 55 kilogram person would cause worse injury than that seen in a 70 kilogram person.
We did not break it down by age, but 90% of our patients, male or female, were between the ages of 15 and 45. I have one question.
Based on one of your slides, you had a large group of patients with lactate levels measured in the emergency department but who died in the emergency department and are reported in the final analyses. What was the gender breakdown of those patients? What was the gender difference in the patients with lactate levels in the emergency department that died in the emergency department so you could not follow them?
Dr. Edwin A. Deitch (Newark, New Jersey): That is an excellent question. What I can tell you is that 55% of the females who died, died within 24 hours, and only about 40% of the males who died, died within 24 hours. We are going back to look at this data because we think there may be more severe injuries in the females that died, or they may have a distribution of injuries that could affect the outcome.
Footnotes
Supported by NIH Grant P50 GM069790.
Presented at the 127th Annual Meeting of the American Surgical Association.
Reprints: Edwin A. Deitch, MD, Department of Surgery, New Jersey Medical School, MSB 506, 185 South Orange Avenue, Newark, New Jersey 07103. E-mail. edeitch@umdnj.edu.
REFERENCES
- 1.Ananthakrishnan P, Deitch EA. Gut origin sepsis and MODS: the role of sex hormones in modulating intestinal and distant organ injury: a review. XX vs XY. 2003;1:108–117. [Google Scholar]
- 2.Angele MK, Wichman M, Eisenmenger S, et al. Immunologic effects of sex hormones following hemorrhagic shock: potential therapeutic applications: a review. XX vs XY. 2003;1:39–45. [Google Scholar]
- 3.Bone RC. Toward an epidemiology and natural history of SIRS. JAMA. 1992;268:3452–3455. [PubMed] [Google Scholar]
- 4.Offner P, Moore E, Biffl W. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–938. [DOI] [PubMed] [Google Scholar]
- 5.Oberholzer A, Keel M, Zellweger R, et al. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–937. [DOI] [PubMed] [Google Scholar]
- 6.Napolitano LM, Greco ME, Rodriguez A, et al. Gender differences in adverse outcomes after blunt trauma. J Trauma. 2001;50:274–280. [DOI] [PubMed] [Google Scholar]
- 7.Wohltmann CD, Franklin GA, Boaz PW, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181:297–300. [DOI] [PubMed] [Google Scholar]
- 8.Rappold JF, Coimbra R, Hoyt DB, et al. Female gender does not protect blunt trauma patients from complications and mortality. J Trauma. 2002;53:436–441. [DOI] [PubMed] [Google Scholar]
- 9.Mostafa G, Huynh T, Sing RF, et al. Gender-related outcomes in trauma. J Trauma. 2002;53:430–435. [DOI] [PubMed] [Google Scholar]
- 10.Croce MA, Fabian TC, Malhotra AK, et al. Does gender difference influence outcome? J Trauma. 2002;53:889–894. [DOI] [PubMed] [Google Scholar]
- 11.Gannon CJ, Pasquale M, Tracy JK, et al. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410–414. [DOI] [PubMed] [Google Scholar]
- 12.George RL, McGwin G, Windham ST, et al. Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19:28–32. [DOI] [PubMed] [Google Scholar]
- 13.Frink M, Pape HC, Griensven, et al. Influence of sex and age on MODS and cytokines after multiple injuries. Shock. 2007;27:151–156. [DOI] [PubMed] [Google Scholar]
- 14.Osborn TM, Tracy JK, Dunne JR, et al. Epidemiology of sepsis in patients with traumatic injury. Crit Care Med. 2004;32:2234–2240. [DOI] [PubMed] [Google Scholar]
- 15.Caruso JM, Deitch EA, Xu DZ, et al. Gut injury and gut-induced lung injury after trauma-hemorrhagic shock is gender and estrus cycle specific in the rat. J Trauma. 2003;55:531–539. [DOI] [PubMed] [Google Scholar]
- 16.Spratt D, Longcope C, Cox P. Differential changes in serum concentrations of androgens and estrogens in postmenopausal women with acute illness. J Clin Endocrinol Metab. 1993;76:1542–1547. [DOI] [PubMed] [Google Scholar]
- 17.Fourrier F, Jallot A, Leclerc L, et al. Sex steroid hormones in circulatory shock, sepsis syndrome and septic shock. Circ Shock. 1994;43:171–178. [PubMed] [Google Scholar]
- 18.Woolf PD, Hamill RW, McDonald JV, et al. Transient hypogonadotropic hypogonadism caused by critical illness. J Clin Endocrinol Metab. 1988;60:444–450. [DOI] [PubMed] [Google Scholar]
- 19.Lavery RF, Livingston DH, Tortella BJ, et al. The utility of venous lactate to triage injured patients in the trauma center. J Am Coll Surg. 2000;190:656–664. [DOI] [PubMed] [Google Scholar]
- 20.Sauaia A, Moore F, Moore EE, et al. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45:291–301. [DOI] [PubMed] [Google Scholar]
- 21.Davis J, Kaups K. Base deficit in the elderly: a marker of severe injury and death. J Trauma. 1988;45:873–877. [DOI] [PubMed] [Google Scholar]
- 22.Abramson D, Scalea TO, Hitchcock R. Lactate clearance and survival following injury. J Trauma. 1993;35:584–589. [DOI] [PubMed] [Google Scholar]
- 23.Rixen D, Siegel JH. Metabolic correlates of oxygen debt predict posttrauma early acute respiratory distress syndrome and the related cytokine response. J Trauma. 2000;49:392–402. [DOI] [PubMed] [Google Scholar]
- 24.Bakker J, Coffernils M, Leon M, et al. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in septic shock. Chest. 1991;99:956–962. [DOI] [PubMed] [Google Scholar]
- 25.McKinley BA, Kozar RA, Cocanour CS, et al. Standardized trauma resuscitatrion: female hearts respond better. Arch Surg. 2002;137:578–584. [DOI] [PubMed] [Google Scholar]
- 26.Christeff N, Benassayag C, Carli-Vielle C, et al. Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J Steroid Biochem. 1888;29:435–440. [DOI] [PubMed] [Google Scholar]
- 27.Angstwirm MWA, Gaertner R, Schopohl J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med. 2005;33:2786–2793. [DOI] [PubMed] [Google Scholar]
- 28.Luppa P, Munker R, Nagel D, et al. Serum androgens in intensive-care patients: correlations with clinical findings. Clin Endrocrinol. 1991;34:305–310. [DOI] [PubMed] [Google Scholar]
- 29.Schroder J, Kahlke V, Staubach KH, et al. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. [DOI] [PubMed] [Google Scholar]
- 30.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. [DOI] [PubMed] [Google Scholar]
- 31.Jarrar D, Wang P, Chaudry IH, et al. Mechanisms of the salutary effects of dehydroepiandrosterone after trauma-hemorrhage; direct or indirect effects on cardiac and hepatocellular functions? Arch Surg. 2000;135:416–423. [DOI] [PubMed] [Google Scholar]
- 32.Remmers DE, Wang P, Chaudry IH, et al. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919–H2925. [DOI] [PubMed] [Google Scholar]
- 33.Ananthakrishnan P, Cohen DB, Deitch EA, et al. Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137:56–65. [DOI] [PubMed] [Google Scholar]
- 34.Ba ZF, Kuebler JF, Rue LW, et al. Gender dimorphic tissue perfusion response after acute hemorrhage and resuscitation: role of vascular endothelial cell function. Am J Physiol Heart Circ Physiol. 2003;284:H21262–H2169. [DOI] [PubMed] [Google Scholar]
- 35.Toth B, Yokoyama Y, Chaudry IH, et al. Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch Surg. 2003;138:1375–1382. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Davidge ST. Effect of estrogen replacement on vasoconstrictor responses in rat mesenteric arteries. Hypertension. 1992;34:1117–1122. [DOI] [PubMed] [Google Scholar]
- 37.Dubney RK, Jackson EK, Keller PJ, et al. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37:640–644. [DOI] [PubMed] [Google Scholar]
- 38.Davidge ST, Zhang Y. Estrogen replacement suppresses a prostaglandin H synthase-dependent vasoconstrictor in rat mesenteric arteries. Circ Res. 1998;83:388–395. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda K, Ruff A, Morinelli TA, et al. Testosterone increases thromboxane A2 receptor density and responsiveness in rat aortas and platelets. Am J Physiol. 1994;267:H887–H893. [DOI] [PubMed] [Google Scholar]
- 40.Chen YF, Naftilan AJ, Oparil S. Andogen-dependent angiotensinogen and rennin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. [DOI] [PubMed] [Google Scholar]
- 41.Deitch EA. Multiple organ failure: pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angele MK, Schwacha MG, Ayala A, et al. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. [DOI] [PubMed] [Google Scholar]
- 43.Deitch EA, Ananthakrishnan P, Cohen DB, et al. Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am J Physiol Heart Circ Physiol. 2006;291:H1456–H1465. [DOI] [PubMed] [Google Scholar]
- 44.Senthil M, Brown M, Xu DZ, et al. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple organ dysfunction syndrome: validating studies in a porcine model. J Trauma. 2006;60:958–967. [DOI] [PubMed] [Google Scholar]
- 45.Sarin EL, Moore EE, Moore JB, et al. Systemic neutrophil priming by lipid mediators in post-shock mesenteric lymph exists across species. J Trauma. 2004;57:950–954. [DOI] [PubMed] [Google Scholar]
- 46.Deitch EA, Forsythe R, Anjaria D, et al. The role of lymph factors in lung injury, bone marrow suppression and endothelial cell dysfunction in a primate model of trauma-hemorrhagic shock. Shock. 2004;22:221–228. [DOI] [PubMed] [Google Scholar]
- 47.Deitch EA, Feketeova E, Adams JM, et al. Lymph from a primate baboon trauma hemorrhagic shock model activates human neutrophils. Shock. 2006;25:460–463. [DOI] [PubMed] [Google Scholar]