Abstract
Objective and Summary Background Data:
Postburn long-term oxandrolone treatment improves hypermetabolism and body composition. The effects of oxandrolone on clinical outcome, body composition, endocrine system, and inflammation during the acute phase postburn in a large prospective randomized single-center trial have not been studied.
Methods:
Burned children (n = 235) with >40% total body surface area burn were randomized (block randomization 4:1) to receive standard burn care (control, n = 190) or standard burn care plus oxandrolone for at least 7 days (oxandrolone 0.1 mg/kg body weight q.12 hours p.o, n = 45). Clinical parameters, body composition, serum hormones, and cytokine expression profiles were measured throughout acute hospitalization. Statistical analysis was performed by Student t test, or ANOVA followed by Bonferroni correction with significance accepted at P < 0.05.
Results:
Demographics and clinical data were similar in both groups. Length of intensive care unit stay was significantly decreased in oxandrolone-treated patients (0.48 ± 0.02 days/% burn) compared with controls (0.56 ± 0.02 days/% burn), (P < 0.05). Control patients lost 8 ± 1% of their lean body mass (LBM), whereas oxandrolone-treated patients had preserved LBM (+9 ± 4%), P < 0.05. Oxandrolone significantly increased serum prealbumin, total protein, testosterone, and AST/ALT, whereas it significantly decreased α2-macroglobulin and complement C3, P < 0.05. Oxandrolone did not adversely affect the endocrine and inflammatory response as we found no significant differences in the hormone panels and cytokine expression profiles.
Conclusions:
In this large prospective, double-blinded, randomized single-center study, oxandrolone shortened length of acute hospital stay, maintained LBM, improved body composition and hepatic protein synthesis while having no adverse effects on the endocrine axis postburn, but was associated with an increase in AST and ALT.
The current investigation was set up to determine the effects of oxandrolone on clinical, metabolic, endocrinologic, and inflammatory markers during the immediate acute phase postburn in a large prospective, single center, double-blinded, randomized, controlled study.
Severe burn injuries induce a hypermetabolic response, which leads to endocrinologic dysfunction, immune compromise, catabolism, and hyper inflammation.1–4 Stress-related hormones such as catecholamines, cortisol, and glucagons drive this response and are markedly elevated postburn.2–6 These changes result in a hypercatabolic state, which is characterized by a significant loss of lean body mass (LBM), even in the fed state, persistent muscle weakness, tachycardia, early fatigue with normal activity, and growth arrest.2–4,7–11 The loss of total nitrogen from the skeletal muscle may be as much as 10- to 15-fold greater in the burned patient than in a healthy-fasted volunteer.11 These drastic changes are most pronounced immediately postburn and up to 6 to 8 weeks postburn until the patient is discharged from the unit.1,12–15 Hypermetabolism, however, has been shown to persist over 2 years leaving the patient disabled and preventing them from reintegration into society.14,15 Attenuation of these responses has been investigated and shown to be beneficial in terms of improved rehabilitation and accelerated reintegration of the patient into society.2,6,16
One of the agents that have been shown to be effective is oxandrolone.17–20 Oxandrolone, an oral synthetic testosterone analogue, has been successfully used in children with Turner syndrome and other pathologic delays in growth, cachexia, alcoholic hepatitis, and in patients with AIDS-associated wasting myopathy.21–24 Compared with testosterone, oxandrolone has minimal virilizing activity.25,26 In burned children and adults, oxandrolone has shown promising results in terms of weight gain and urinary nitrogen balance during the acute phase postburn.18,20 oxandrolone further improved body composition and strength in severely burned children during the 12 months of treatment.15,18 Recently, a multicenter study showed that oxandrolone significantly shortened length of hospital stay in 81 severely burned adults.27 Taking these data together, it appears that oxandrolone is a beneficial treatment option for severely burned patients; however, there is no large prospective single center trial on the effect of oxandrolone on clinical outcome. Furthermore, the underlying mechanisms responsible for the improved clinical outcome are not fully understood. As the peak of the hypermetabolic and inflammatory responses occurs immediately after burn,1,2,6,12 the current study was designed to investigate the effect of oxandrolone on clinical, metabolic, endocrinologic, and inflammatory markers during the immediate acute phase postburn.
PATIENTS AND METHODS
Thermally injured children over a time period of 8 years who were admitted to our burn unit and required at least 1 surgical intervention were included in this study. Two hundred thirty-five severely burned children with >40% total body surface area (TBSA) burn were enrolled in the study and randomized (block randomization 4:1) to receive standard burn care (control, n = 190) or standard burn care plus oxandrolone (oxandrolone, n = 45). When patients were admitted within the first 24 hours, postburn patients were resuscitated according to the Galveston formula with 5000 mL/m2 TBSA burned + 2000 mL/m2 TBSA lactated Ringer’s solution given in increments over the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision and the wounds covered with available autograft skin with allograft used to cover any remaining open areas. After the first operative procedure, it took 5 to 10 days until the donor site was healed and patients were then taken back to the operation theater. This procedure was repeated until all open wound areas were covered with autologous skin material.
All 235 patients received placebo for the first 7 days after hospital admission, which is the 6th day after their first operation. According to their randomization, patients then were given oxandrolone or placebo. Oxandrolone was administered at 0.1 mg/kg body weight q.12 hours p.o, n = 45 and drug administration was monitored prospectively by pharmacists from our institute. Oxandrolone or placebo was given throughout the entire hospital course until patients were discharged from the intensive care unit (ICU). This study was limited to acute hospitalization; therefore, we did not include any long-term oxandrolone administration. Duration of oxandrolone administration was similar for all oxandrolone-treated patients, which was approximately 30 days.
All patients underwent the same nutritional treatment according to a standardized protocol. We used the Galveston formulas, Galveston Infant, Galveston Revised, and Galveston Adolescent. The formula change with age based on the body surface alterations that occur with growth. Roughly, the intake is calculated as 1500 kcal/m2 body surface + 1500 kcal/m2 area burn or we assessed the caloric need by measuring the resting energy expenditure (REE) and multiplied by 1.4 as previously published.28 The nutritional route of choice in our patient population was enteral nutrition, and none of the patients in this study received parenteral nutrition. The amount of calories was the same in both groups.
Patient demographics (age, date of burn and admission, sex, burn size and depth of burn) and concomitant injuries, such as inhalation injury, sepsis, morbidity, and mortality were recorded. Sepsis was defined as a blood culture or pathologic tissue identifying the pathogen during hospitalization or at autopsy, in combination with at least 3 of the following: leucocytosis or leucopenia (>12,000 or <4000), hyperthermia or hypothermia (>38.5 or <36.5°C), tachycardia (>150 BPM in children), refractory hypotension (systolic BP <90 mm Hg), thrombocytopenia (platelets <50,000/mm3), hyperglycemia (serum glucose >240 mg/dL), and enteral feeding intolerance (residuals >200 mL/h or diarrhea >1 L/d) as previously published.29–31 Wound healing was evaluated from time of donor site healing and thus time between operative interventions.
Indirect Calorimetry
As part of our routine clinical practice, all patients underwent REE measurements within 1 week after hospital admission and weekly thereafter during their acute hospitalization. This and subsequent measurements of REE were performed between midnight and 5 am while the patients were asleep and receiving continuous feeding. REE was measured using a Sensor-Medics Vmax 29 metabolic cart (Yorba Linda, CA) as previously published.13 The REE was calculated from the oxygen consumption and carbon dioxide production by equations described by Weir and 1 recent publication.13 Measured values were compared with predicted norms based upon the Harris-Benedict equation and to body mass index (BMI).13 For statistical comparison, energy expenditure was expressed both as absolute REE, as the percentage of the basal metabolic rate predicted by the Harris-Benedict equation, and as actual REE normalized to BMI.
Body Composition
Height and body weight were determined clinically 5 days after admit and at discharge. Total LBM, fat, bone mineral density (BMD), and bone mineral content (BMC) were measured by dual energy x-ray absorptiometry (DEXA). An Hologic model QDR-4500W DEXA (Hologic, Waltham, MA) was used to measure body composition as previously published.14,15
Serum Hormones, Proteins, and Cytokines
Blood or urine was collected from the burn patients at the time of admission, preoperatively, and 5 days post operatively for 4 weeks for serum hormone, protein, cytokine, and urine hormone analysis. Blood was drawn in a serum-separator collection tube and centrifuged for 10 minutes at 1320 rpm, the serum was removed and stored at −70°C until assayed.
Serum hormones and acute phase proteins were determined using HPLC and ELISA techniques. The Bio-Plex Human Cytokine 17-Plex panel was used with the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA) to profile expression of seventeen inflammatory mediators [IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), interferon-gamma (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 beta (MIP-1β), and TNF. The assay was performed according to the manufacturer’s instructions as previously published.1,12
Urine cortisol was determined by standard laboratory techniques, counting for urine amount, creatinine, and creatinine clearance.
Cardiac and Liver Changes
M-Mode echocardiograms were completed as follows: at the time of the study, none of the patients presented with or previously suffered from other concomitant diseases affecting cardiac function, such as diabetes mellitus, coronary artery disease, long standing hypertension, or hyperthyroidism. Study variables included resting cardiac output (CO), cardiac index (CI), stroke volume (SV), resting heart rate (HR), and left ventricular ejection fraction. SV and CO were adjusted for body surface area and expressed as indexes. All ultrasound measurements were made with the Sonosite Titan echocardiogram, with a 3.5 MHz transducer. Recordings were performed with the subjects in a supine position and breathing freely. M-Mode tracings were obtained at the level of the tips of the mitral leaflets in the parasternal long axis position and measurements were performed according to the American Society of Echocardiography recommendations. Left ventricular volumes determined at end diastole and end systole were used to calculate ejection fraction (EF), SV, CO and CI. Three measurements were performed and averaged for data analysis.
Ultrasound measurements, in this study, were made with the HP Sonos 100 CF echocardiogram (Hewlett Packard Imaging Systems, Andover, MA). The liver was scanned using an Eskoline B-scanner, a modified HP diagnostic sounder 7214 A, and a modified 3.5 MHz transducer probe. Liver size was calculated using the previously published formula and actual size was then compared with predicted size.32
Strength Measurements
Strength testing was conducted in controls and oxandrolone-treated patients between discharge and 6 months postburn before the patients underwent exercise training using a Biodex System-3 dynamometer (Shirley, NY). The isokinetic test was performed on the dominant leg extensors and tested at an angular velocity of 150°/s. This speed was chosen as it was well tolerated (compared with lower or higher angular speeds) by the children across all ages and all groups. The patients were seated and their position stabilized with a restraining strap over the midthigh, pelvis, and trunk in accordance to the Biodex System-3 Operator’s Manual. All patients were familiarized with the Biodex test in a similar manner. First, the administrator of the test demonstrated the procedure. Second, the test procedure was explained to patients, and third, patients were allowed to practice the actual movement during 3 submaximal repetitions without load as warm-up. More repetitions were not allowed to prevent the onset of fatigue. The anatomic axis of the knee joint was aligned with the mechanical axis of the dynamometer before the test. After the 3 submaximal warm-up repetitions, 10 maximal voluntary muscle contractions (full extension and flexion) were performed. The maximal repetitions were performed consecutively without rest in between. Three minutes of rest was given to minimize the effects of fatigue and the test was repeated. Values of peak torque were calculated by the Biodex software system. The highest peak torque measurement between the 2 trials was selected. Peak torque was corrected for gravitational moments of the lower leg and the lever arm.
Ethics and Statistics
The study was reviewed and approved by the Institutional Review Board, of the University Texas Medical Branch, Galveston, Texas. Before the study, each subject, parent or child’s legal guardian had to sign a written informed consent form. Analysis of variance (ANOVA) with post hoc Bonferroni correction, unpaired Student t test (1 tail and 2 tail), χ2 analysis, and Mann-Whitney tests were used where appropriate. Data are expressed as means ± SEM or percentages, where appropriate. Significance was accepted at P < 0.05.
RESULTS
Demographics
Two hundred thirty-five severely burned children were included in the present study. Patients’ demographics are shown in Table 1. There was no significant difference in age, gender distribution, TBSA burn, third-degree burn, and time from burn to hospital admit between controls and oxandrolone (Table 1). There was no difference between groups in the incidence of inhalation injury, major and minor infections, mortality, and nutritional intake (Table 1). Length of hospital stay was 31 ± 2 days in controls and 28 ± 2 days in oxandrolone-treated patients. When length of stay was normalized to TBSA burn, oxandrolone-treated patients had a significant shorter ICU stay when compared with controls, P < 0.05 (Table 1). The number of operations (OR) was not significantly different between groups; however, time between ORs was significantly decreased in the oxandrolone group when compared with controls, implying an increased wound healing rate in patients treated with oxandrolone, P < 0.05 (Table 1).
TABLE 1. Patient Demographics
Indirect Calorimetry
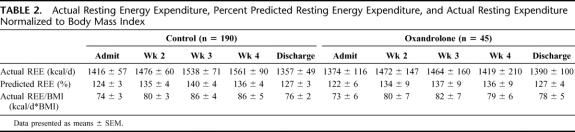
Actual REE, percent predicted REE, and actual REE normalized to BMI were determined (Table 2). Confirming previous studies, actual REE, percent predicted REE, and actual REE/BMI increased postburn and remained elevated throughout acute hospital stay. There was no significant difference between controls and oxandrolone in actual REE, percent predicted REE, and actual REE/BMI (Table 2).
TABLE 2. Actual Resting Energy Expenditure, Percent Predicted Resting Energy Expenditure, and Actual Resting Expenditure Normalized to Body Mass Index
Body Composition
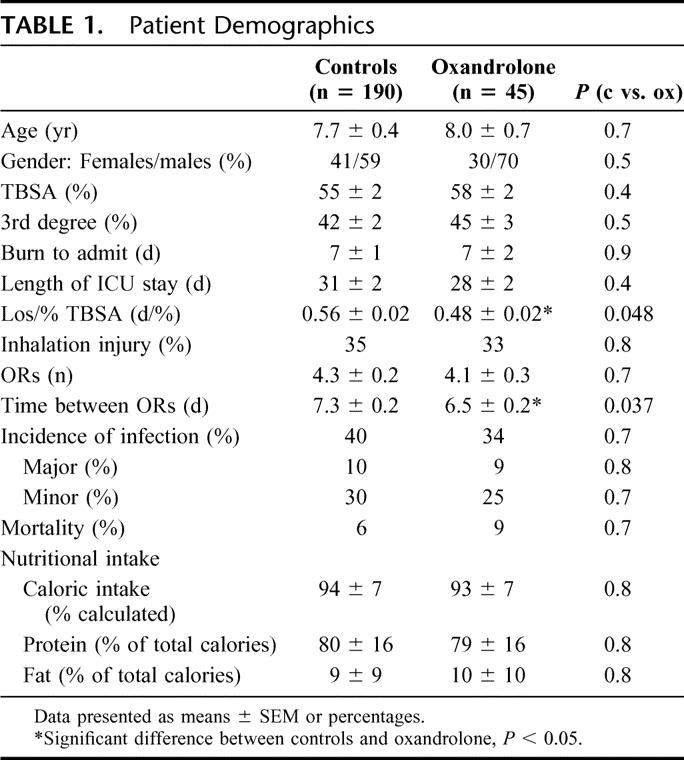
There were distinct differences in body composition between controls and oxandrolone-treated patients. Both groups demonstrated a decrease in BMC (controls: −5 ± 2%, vs. oxandrolone: −8 ± 6%) and BMD (controls: −3 ± 2% vs. oxandrolone: −6 ± 6%) from admit to discharge with no differences between controls and oxandrolone. Both groups had an increase in total whole body fat from admit to discharge with no difference between controls and oxandrolone (Fig. 1). Control patients lost about 8% of their whole body mass and 9% to 10% of their LBM during acute hospitalization (Fig. 1). Oxandrolone treatment reversed this catabolism and preserved body and muscle mass by increasing total whole body mass and LBM (+8%), which was significantly different from controls, P < 0.05 (Fig. 1).
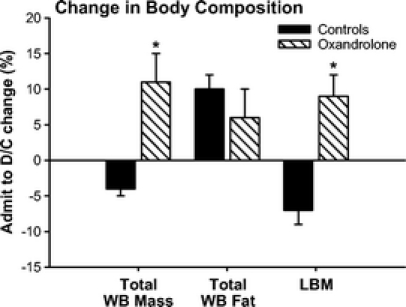
FIGURE 1. Percent change in body composition from admit to discharge. Both groups had an increase in total whole body fat from admit to discharge with no difference between controls and oxandrolone. Oxandrolone treatment reversed catabolism and preserved body and muscle mass by increasing total whole body mass and lean body mass when compared with controls. *Significant difference between controls versus oxandrolone, P < 0.05.
Serum Hormones, Proteins, and Cytokines
Serum IGF-I and IGFBP-3 decreased immediately after burn in both groups and remained decreased throughout the entire study period (Fig. 2). Oxandrolone treatment had no effect on serum IGF-I and IGFBP-3 (Fig. 2). Serum GH markedly decreased in both groups with no significant difference between controls and oxandrolone (Fig. 2).
FIGURE 2. Serum IGF-I and IGFBP-3 decreased immediately after burn in both groups with significant difference between controls and oxandrolone. Serum GH was in the normal range immediately postburn, but started to decrease 2 to 7 days postburn. There was no difference between controls and oxandrolone. Serum estrogen (β-estradiol) and testosterone decreased in both groups postburn. Oxandrolone had no effect on serum β-estradiol, but significantly increased serum testosterone at various time points compared with controls. *Significant difference between controls versus oxandrolone, P < 0.05. Dashed lines represent upper and lower limit of the normal range.
Sex hormones, estrogen (β-estradiol), progesterone, and testosterone decreased in both groups postburn. Oxandrolone had no effect on serum β-estradiol (Fig. 2). Oxandrolone significantly increased serum testosterone at various time points compared with controls, P < 0.05 (Fig. 2).
Serum liver enzymes AST and ALT increased postburn in controls but decreased to the normal range at 17 to 22 days postburn (Fig. 3). Oxandrolone caused a significant increase in serum AST and ALT beginning at 17 days postburn, P < 0.05 (Fig. 3). However, liver function was not impaired as we found significantly increased constitutive hepatic protein levels with oxandrolone treatment. Oxandrolone increased total serum proteins and prealbumin, whereas it decreased acute phase proteins α2-macroglobulin and complement C3, P < 0.05 (Fig. 4). Oxandrolone significantly decreased serum-free fatty acids compared with controls, P < 0.05 (Fig. 4). Oxandrolone had no effect on serum C-reactive protein, α1-acid glycoprotein, transferrin, retinol-binding protein, and triglycerides.
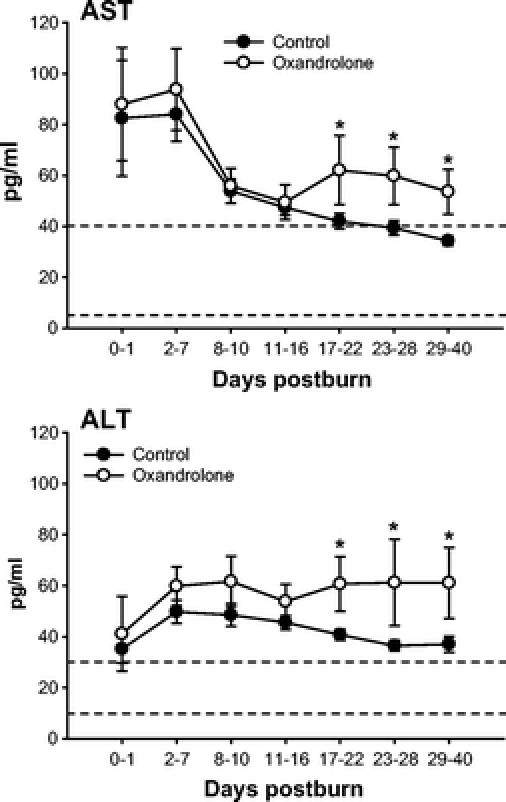
FIGURE 3. Serum liver enzymes AST and ALT increased postburn but decreased to the normal range. Oxandrolone caused a significant increase in serum AST and ALT beginning at 17 days postburn. *Significant difference between controls versus oxandrolone, P < 0.05. Dashed lines represent upper and lower limit of the normal range.
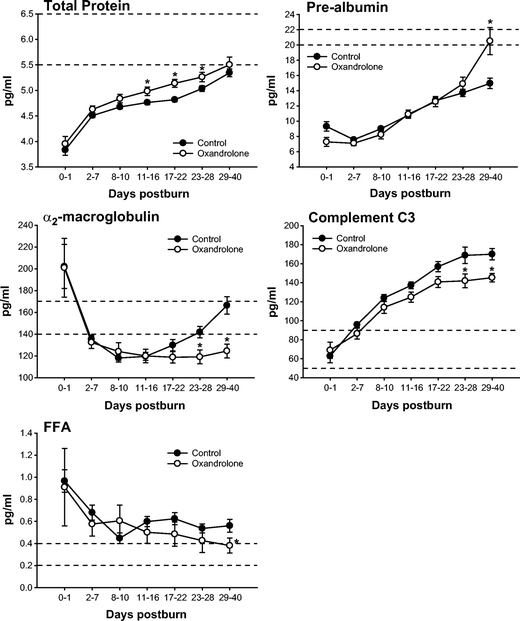
FIGURE 4. Serum constitutive hepatic and acute phase proteins. Oxandrolone increased total serum proteins and prealbumin, whereas it decreased acute phase proteins α2-macroglobulin and complement C3. Oxandrolone significantly decreased serum free fatty acids. *Significant difference between controls versus oxandrolone, P < 0.05. Dashed lines represent upper and lower limit of the normal range.
In terms of serum cytokines, we confirmed previous studies in which we showed that burn causes a marked inflammatory response. There was no difference between controls and oxandrolone-treated patients in the inflammatory response postburn (Fig. 5).
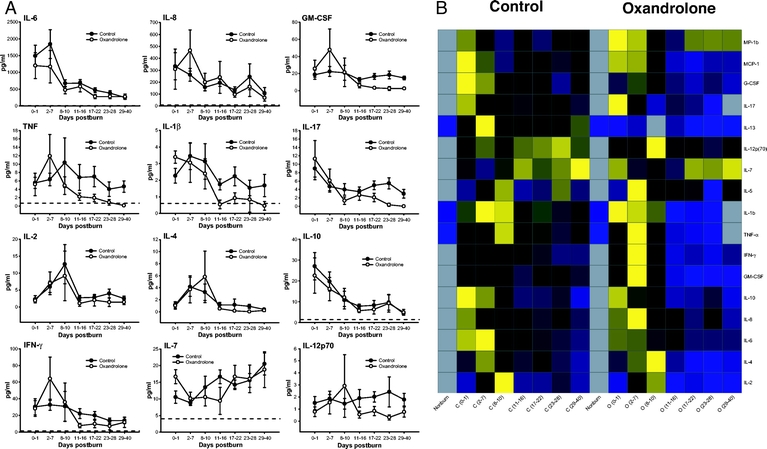
FIGURE 5. A, The effect of oxandrolone on pro- and anti-inflammatory cytokines. Oxandrolone did not have any significant effect on the inflammatory response. Dashed lines represent upper and lower limit of the normal range. Normal ranges: IL-6: <8 pg/mL, IL-8: <7 pg/mL, GM-CSF: 0 pg/mL, TNF: <0.7 pg/mL, IL-1β: <0.8 pg/mL, IL-17: 0 pg/mL, IL-2: 0 pg/mL, IL-4: 0 pg/mL, IL-10: <1.4 mg/dL, IFN-γ: <1.3 pg/mL, IL-7: <4 pg/mL, IL-12 p70: 0 pg/mL. B, Heat map comparing normal (noninjured, nonburned children), controls at each time point [C (0–1 day postburn), C (2–7 days postburn), C (8–10 days postburn), C (11–16 days postburn), C (17–22 days postburn), C (23–28 days postburn), and C (29–40 days postburn)], and normal and oxandrolone at each time point [O (0–1 day postburn), O (2–7 days postburn), O (8–10 days postburn), O (11–16 days postburn), O (17–22 days postburn), O (23–28 days postburn), and O (29–40 days postburn)] serum cytokine protein expression profiles. Values are log10 (average cytokine concentration, pg/mL); the color range for each cytokine is based on the detected values with blue indicating lower levels, yellow indicating highest levels, and black in the middle. Gray squares indicate that no expression was detected.
Cardiac and Liver Changes
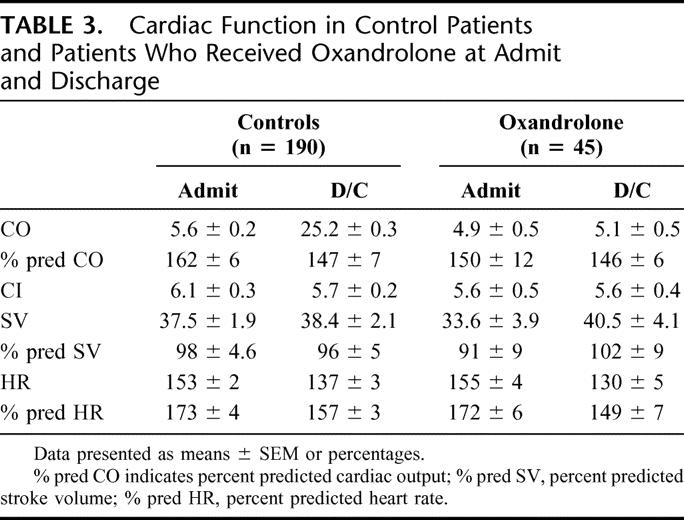
Analysis of CO, predicted CO, CI, SV, predicted SV, HR, predicted HR, CI, and central venous pressure showed no differences between controls and oxandrolone-treated patients (Table 3).
TABLE 3. Cardiac Function in Control Patients and Patients Who Received Oxandrolone at Admit and Discharge
Immediately after burn, liver size (length: controls 4 ± 5% vs. oxandrolone: 5 ± 5%) and weight (weight: controls: 55 ± 22% vs. oxandrolone: 43 ± 27%) increased in both groups. There was no significant difference between controls and oxandrolone in percent change in liver length and liver weight from admit to discharge.
Strength Measurements
Thirty-nine control patients (age: 11.4 ± 0.24 years, TBSA: 54 ± 4%, third-degree TBSA: 43 ± 3%, 34 males and 5 females) and 19 patients treated with oxandrolone (age: 12.0 ± 0.7 years, TBSA: 53 ± 3%, third-degree TBSA: 33 ± 5%, 16 males and 3 females) were assessed for muscle strength. Muscle strength was assessed after discharge before our exercise rehabilitation program was started, which is usually between 3 and 6 months postburn. Muscle strength decreased in both groups compared with normal nonburned children. However, the mean muscle strength (peak torque) was significantly higher (2 folds) in the oxandrolone group when compared with the control group, P < 0.05 (Fig. 6).
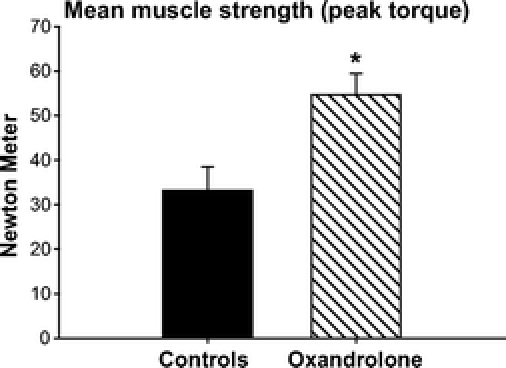
FIGURE 6. Muscle strength was tested before the beginning of the rehabilitation-exercise program. Burn injury caused significant decrease in muscle strength (peak torque from normal, nonburned children: 95 ± 5 N m).46 oxandrolone almost doubled muscle strength and significantly increased peak torque compared with controls. *Significant difference between controls versus oxandrolone, P < 0.05.
DISCUSSION
Oxandrolone, a testosterone analogue, has been used for cachectic and protein wasting diseases to improve catabolism and clinical outcomes in various pathophysiological states. Burns induce a marked inflammatory and hypermetabolic response in which the metabolic rate is extremely high and energy requirements are immense and met by the mobilization of proteins and amino acids.33 Increased protein turnover, degradation and negative nitrogen balance are characteristics of this severe critical illness.33 As a consequence, the structure and function of essential organs, such as skeletal muscle, skin, immune system, and cellular membrane transport functions are compromised.7,34 This compromise can lead to multi-organ dysfunction or even death. Therefore, administration of various anabolic agents has been used in an attempt to attenuate the protein loss and wasting process. Testosterone as an anabolic substance was clinically introduced in men when it was found to be markedly decreased poststress and postburn. Testosterone attenuated muscle protein catabolism, but its administration was associated with increased virilization.25,26 Therefore, oxandrolone a synthetic testosterone analogue with much less virilizing activity was introduced.25,26 oxandrolone was tested in various muscle wasting diseases, such as cachexia, alcoholic hepatitis, and in patients with AIDS wasting myopathy.21–24 In burned children and adults, oxandrolone has shown promising results in terms of weight gain and urinary nitrogen balance during the acute phase postburn.18,20 Oxandrolone further improved body composition and strength in severely burned children during 12 months of treatment.15,18 Recently, a multicenter study showed that oxandrolone significantly shortened length of hospital stay in 81 severely burned adults.27 Taking these data together, it appears that oxandrolone is a beneficial treatment option for severely burned patients; however, to date, no large prospective single-center trial on the effect of oxandrolone on clinical outcomes during acute hospitalization was conducted.
In the present study, we found that oxandrolone administration during acute hospitalization significantly decreased length of hospital stay. This data is in agreement with the recent multicenter study in burned adults.27 Based on our data, we suggest that decreased length of ICU stay is due to a shortened interval between operations. We found that the time interval between ORs are 7.3 ± 0.2 days in controls patients whereas it is 6.5 ± 0.2 days in oxandrolone-treated patients. We did not determine the rate of wound healing or donor site healing in this study; however, a decreased time interval between ORs would imply that oxandrolone-treated patients have an improved wound or donor site healing. It is not clear whether increased wound healing is due to a direct cell-specific effect of oxandrolone or an indirect effect due to improved LBM and thus protein synthesis and availability.35–37
In the present study, we confirmed previous studies in others and we have shown that a severe burn causes a marked increase in cardiac work and inflammation, which leads to a massive hypermetabolic response reflected by increased REE. We found no difference between controls and oxandrolone-treated patients during the acute hospitalization in REE, predicted REE, CO, CI, SV, or HR. In the present study, we further showed that burn patients are hypermetabolic and lose approximately 5% to 8% of their total body weight during acute hospitalization and 10% of their LBM. This drastic catabolic response was abrogated with oxandrolone. We showed that oxandrolone preserved whole body and lean body muscle mass. As the inflammatory and hypermetabolic response was not significantly different between the 2 groups, we suggest that oxandrolone does not improve muscle catabolism via these mechanisms. Oxandrolone itself acts directly on tissue via the androgen receptor and by activating down-stream molecules.38–40 Recently, it was shown that oxandrolone suppresses glucocorticoid action via crosstalk between the androgen receptor and glucocorticoid receptor.40 Glucocorticoids have been described as one of the major hormones responsible for proteolysis.5,41–43 Glucocorticoids also increase lipolysis and causes fat accumulation.44,45 Suppressing glucocorticoid activity is a possible molecular biologic link by which oxandrolone affect catabolism and decreases lipolysis, shown by decreased free fatty acid concentrations.
We found in the present study that oxandrolone improved body weight during acute hospitalization. Whereas control patients lost 8% to 10% of their body weight, oxandrolone-treated patients gained body weight during acute hospitalization. As we found no difference in BMC, BMD, and whole body fat, but a significant increase in LBM with oxandrolone administration, we conclude that increased body weight was due to increased lean body muscle mass and not due to fat or bone mass. More importantly, we found that increased lean body muscle was functional muscle mass as we demonstrated that oxandrolone significantly increased peak torque and maximal strength, which was almost 2 folds, in our oxandrolone-treated patient population. Increased functional muscle mass and total body weight is associated with improved mobilization, daily activity, and thus reintegration of the burn victim into society.46–49 Therefore, oxandrolone appears to be a beneficial treatment during acute hospitalization.
A critique is that the DEXA method is not specific and also determines water content and not only LBM. We conducted a study in which we compared DEXA with the K count that is specific for muscle mass. We found in over 100 pediatric burned patients that DEXA and K count show a very strong positive correlation, implying that DEXA determines changes in LBM (unpublished data). In addition, we measured LBM the earliest 7 days post admit, which was for this patient population 10 to 14 days postburn. From our experience, major fluid shifts occur immediately postburn and last for 5 to 7 days postburn. At our institute, we consider a patient 5 days postburn at the patients’ dry weight as the major fluid shifts have passed. Based on the validation of the DEXA and the time we conducted the DEXA studies, we suggest that we detected changes in LBM and not water concentrations. However, as we did not measure body water content, we cannot make a definitive statement, which is a limitation to this study. In future studies, we will include body water content measurements.
A possible concern associated with oxandrolone administration is an increase in liver enzymes, a phenomenon that has been described also by other groups.22,50 During our study period, oxandrolone significantly increased serum AST and ALT, which occurred 2 to 3 weeks after oxandrolone treatment was started. Serum AST and ALT are normally considered markers for hepatic damage, and therefore, one may suggest that oxandrolone cause some degree of liver damage; however, we further showed that oxandrolone decreased hepatic acute phase protein concentration whereas it increased constitutive hepatic protein concentration. This indicates an improved hepatic homeostasis and protein production by the liver. This finding was also shown by Thomas and colleagues.51 We also showed that liver size and weight was not significantly different between controls and oxandrolone patients, which indicates that oxandrolone did not cause hepatomegaly. It furthermore appears that oxandrolone did not cause major hepatic damage. We measured serum IGF-I, IGFBP-3, and GH, all of which are mainly synthesized in the liver52 and found no significant difference between controls and oxandrolone-treated patients. These data indicate that liver function maintains intact with the use of oxandrolone, but we suggest that the elevation of liver enzymes should be monitored during the treatment period with oxandrolone.
An unexpected finding in our study was that oxandrolone significantly increased testosterone levels. Our original hypothesis was that oxandrolone would decrease or have no effect on endogenous testosterone levels as previously published.53–55 However, we found that oxandrolone administration significantly increased testosterone levels at several time points postburn. Similar data were found by Malhotra and colleagues56 in growth delayed boys. The authors suggested that oxandrolone has an androgenic action and effect thus stimulating serum LH and testosterone.56 The exact mechanisms by which oxandrolone increases endogenous androgenic hormone production are unknown. In light of the greater percentage of boys in the oxandrolone treatment group, we asked the question whether it is possible that gender predominance influenced the results by virtue of a greater androgenic effect in males than in females. In a subgroup analysis, we examined whether oxandrolone had a greater effect on males versus females for body composition, serum proteins and cytokines, and hormones. We also looked at sex differences in terms of strength. We found, as previously described, that males are different from females, but there was no significant gender effect of oxandrolone for the parameters measured.
In summary, this study is the first of its kind to investigate this anabolic agent during the acute phase postburn in a large single-center randomized double-blinded study. We showed that oxandrolone ameliorated catabolic responses that occur immediately after burn. Oxandrolone shortened the length of hospital stay, which was associated with a decreased time interval between operations, indicating improved wound healing. Although oxandrolone increased hepatic enzymes it did not have any adverse effects on the hypermetabolic, inflammatory, or endocrinologic responses postburn.
Discussions
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): It should be noted that 33 years ago at the meeting of this Association, Dr. Douglas Wilmore and his associates identified the catecholamines as the mediators of post-injury hypermetabolism. Only 4 years later, Dr. Herndon, the senior author of today’s paper, presented his first paper on the metabolic effects of injury at the Surgical Forum. In the intervening years Dr. Herndon’s group has compiled an impressive volume of work that has defined metabolic support regimens that have reduced mortality and enhanced outcomes of severely injured burn patients. Today’s paper is a worthy addition to that aggregate opus.
There are several questions, the answers to which will help us interpret your findings and evaluate your conclusions.
In other studies you have identified a gender-specific difference in response to burn injury that favors girls. Consequently, is it possible that the 11% greater number of boys in the treatment group affected the results since one would anticipate that the androgenic effect might be greater?
You note that parenteral nutrition was preferred but parenteral nutrients were given when needed to meet nutritional goals. Were there more patients in the control group who required parenteral nutrients? If there were, do the differences in weight change reflect, at least in part, differences in calorie and protein intake?
How comparable were fluid intake and output in the 2 groups? Is it possible that the increased body weight in the treatment group merely represents water retention?
Do the effects of oxandrolone persist after treatment ceases, or is long-term androgenic hormone therapy required throughout the up-to-2-year period that you have identified as the duration of post-injury hypermetabolism? Finally, is the treatment effect of your hormonal therapy proportional to metabolic rate, ie, can we realize the biggest bang for our androgenic buck in those patients who will experience the greatest benefit?
Dr. Marc G. Jeschke (Galveston, Texas): In response to gender specific effects of oxandrolone, we did look at males and females in our patient population. We looked at body composition using DEXA and at muscle protein synthesis by stable isotope studies, which are not presented here. There was no difference between the 2 genders in terms of oxandrolone treatment. There is a study by Malhotra et al showing that oxandrolone has an androgenic effect especially when testosterone levels are low. Oxandrolone also stimulates female hormones; therefore, oxandrolone has beneficial effects in males and females, and we suggest that this is the reason why we could not find any difference.
In terms of nutrition, we looked at nutritional intake. Both groups were similar in their percent intake and also their required intake, so there was no difference in the nutrition, the route, or the amount of calories administered.
Regarding the fluid water retention, meaning more fluid on board rather than muscle mass. We did a study presented at the American Burn Association 2 years ago (Cochran et al) where we compared dual x-ray absorptometry with K count. The aim of the study was to correlate DEXA with K count and therefore to differentiate whether water retention is detected by DEXA, or is DEXA specific to determining muscle mass. We found that DEXA correlates very well with the K count, indicating muscle rather then water or fluid retention. We also looked at fluid resuscitation and the amount of fluids in the first 48 hours and there was no difference between the groups.
In answer to your question concerning whether the effects of oxandrolone persist after treatment, what was shown 2 years ago at the American Surgical Association meeting was that when you give oxandrolone for 12 months and then you stop it, the effects diminish and there is a rebound phenomenon in the control patients. There is a beneficial treatment effect of oxandrolone; however, as you pointed out, it ceases more or less when you stop treatment. So, it will be beneficial to continue treatment.
And lastly, when you asked whether the treatment efficacy is proportional to testosterone levels; you are absolutely right. The Malhotra study also delineated that the lower the testosterone levels, the more beneficial the oxandrolone treatment. Therefore, an interesting approach is to identify patients who have very low testosterone levels and give oxandrolone to these patients to achieve a therapeutic benefit.
Dr. Ronald V. Maier (Seattle, Washington): We have also used oxandrolone for critically ill trauma patients in an attempt to create anabolic conditions to enhance recovery. And, while it undoubtedly has a positive effect on muscle strength recovery and on wound healing, one of the detriments we noted with patients with multiple organ failure, particularly lung failure where you do not want to have an excessive anabolic effect, was actually a prolongation of ARDS. This prolongation was presumably caused by increasing the fibrotic process in the long-standing ARDS. I wonder if you have had any observations or concerns regarding conditions where you didn’t want to have excessive responses, such as hypertrophic scarring or excess collagen depositions, or whether any of these patients had organ failure where the anabolic effects were actually detrimental.
Dr. Marc G. Jeschke (Galveston, Texas): In terms of the hypertrophic scar, we did a scar assessment. We did this with blinded observers using the Vancouver Scar Scale. What we found is that oxandrolone improves scar maturation and scar development. We observed less hypertrophic scarring, which was a long-term study, not an acute study. These findings indicate that an early wound closure as shown by our data diminishes or decreases proliferation activity of myofibroblasts and inflammation in the skin.
In terms of your question of multi-organ failure, there was no difference in the patient population in the incidence of multi-organ failure or sepsis. And in terms of lung fibrosis or ARDS, we looked at the ventilator data on these patients and didn’t find any difference between the 2 groups.
Dr. William P. Schecter (San Francisco, California): Do you think the evidence is now strong enough that oxandrolone should be given as a routine by protocol to all burn patients? If so, at what point in the period after a burn should this therapy be instituted?
Dr. Marc G. Jeschke (Galveston, Texas): Our group suggests that oxandrolone should be one of the agents considered as a routine drug for severely burned children. As presented here, oxandrolone has significant clinical benefits during acute hospitalization. However, oxandrolone also has some side effects that need to be considered. As we have shown in this study liver enzymes should be measured frequently.
To answer your question, what should the clinical standard be in severely burned patients? Based on the present and other studies, we suggest that standard therapy should include 3 arms of treatment. Number 1: to achieve tight euglycemic control using insulin administration. Number 2: to add propranolol as an anticatabolic or antistress agent and to reduce cardiac work. Lastly, we suggest adding an anabolic agent, such as oxandrolone, for the benefits in muscle protein synthesis. Oxandrolone should also be initiated very early after burn, and then continued until the patient is no longer hypermetabolic, which is a period of about 12 to 18 to 24 months. So these would be the strategies that we are suggesting and also studying.
Dr. Mark D. Pescovitz (Indianapolis, Indiana): There are never enough studies done in children. So, congratulations and thank you for doing a study in children. I have 2 questions.
You said that there was a change in lean body mass because you assumed that if it was not fat, it was lean body. So the first question is, how did you exclude water as the difference in weight?
And the more important question really gets at doing studies in children. The age range, 8 to 18 or 8 to 17, would have required assent, not consent, from the patients. Clearly, you must have asked for a waiver of assent during the acute period when the patient arrived. How did you handle the continued trial? You had a tremendous number of patients. How were you able to get in the number of patients? And how were you able to maintain them once you were able to get assent from them?
Dr. Marc G. Jeschke (Galveston, Texas): In regards to your question: is it water rather than protein that caused the increase in body mass? We believe that the increase in body weight and lean body mass is due to muscle and not water, because usually by 5 days postburn we suggest that the effect of resuscitation is not present anymore and we call it dry weight. Therefore, long after the burn, water should not play the definitive role anymore. As mentioned above, we correlated the DEXA study to the K count study. We found that DEXA correlates well with the K count implying that the DEXA is very specific and reflects very well when you look at lean body mass, that it is muscle rather than water. So, we strongly suggest it is lean body mass that is increased.
In terms of consent, the standard at our hospital is that when we get patients and they are enrolled in studies, we get consent from the parents or legal guardian immediately. That is usually done immediately when the patient comes to our hospital or within 24 hours. We re-consent when our patients come back, 80 to 90% of all patients have to come back for follow-up checkups and surgeries at various time points, eg, 3, 6, 9, 12, 24, 36 months postburn. We have a very close doctor-patient relationship, so we maintain our patients for a long period of time, which makes it possible for us to have these high patient numbers. In regards to your question about assent, when the older patients are awake, alert, and oriented, we request assent from the patient again.
Footnotes
Supported by grants from the American Surgical Association Foundation, Shriners Hospital for Children (8660, 8760, and 9145), NIGMS (R01-GM56687, T32 GM008256, KO1-HL70451, R01-HD049471, and P50 GM60338), and NIDRR H133A020102.
Reprints: Marc G. Jeschke, MD, PhD, Shriners Hospitals for Children, 815 Market Street, Galveston, Texas 77550. E-mail: majeschk@utmb.edu.
REFERENCES
- 1.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. [DOI] [PubMed] [Google Scholar]
- 2.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. [DOI] [PubMed] [Google Scholar]
- 3.Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–1187. [DOI] [PubMed] [Google Scholar]
- 4.Wilmore DW, Long JM, Mason AD Jr, et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care. 1999;2:201–205. [DOI] [PubMed] [Google Scholar]
- 6.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. [DOI] [PubMed] [Google Scholar]
- 7.Klein GL, Herndon DN, Goodman WG, et al. Histomorphometric and biochemical characterization of bone following acute severe burns in children. Bone. 1995;17:455–460. [DOI] [PubMed] [Google Scholar]
- 8.Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995;126:252–256. [DOI] [PubMed] [Google Scholar]
- 9.Klein GL, Langman CB, Herndon DN. Persistent hypoparathyroidism following magnesium repletion in burn-injured children. Pediatr Nephrol. 2000;14:301–304. [DOI] [PubMed] [Google Scholar]
- 10.Klein GL, Wolf SE, Goodman WG, et al. The management of acute bone loss in severe catabolism due to burn injury. Horm Res. 1997;48(suppl 5):83–87. [DOI] [PubMed] [Google Scholar]
- 11.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–395. [DOI] [PubMed] [Google Scholar]
- 12.Finnerty CC, Herndon DN, Chinkes DL, et al. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007;27:4–9. [DOI] [PubMed] [Google Scholar]
- 13.Mlcak RP, Jeschke MG, Barrow RE, et al. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Przkora R, Barrow RE, Jeschke MG, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60:968–971. [DOI] [PubMed] [Google Scholar]
- 15.Przkora R, Jeschke MG, Barrow RE, et al. Metabolic and hormonal changes of severely burned children receiving long-term oxandrolone treatment. Ann Surg. 2005;242:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herndon DN. Nutritional and pharmacological support of the metabolic response to injury. Minerva Anestesiol. 2003;69:264–274. [PubMed] [Google Scholar]
- 17.Barrow RE, Dasu MR, Ferrando AA, et al. Gene expression patterns in skeletal muscle of thermally injured children treated with oxandrolone. Ann Surg. 2003;237:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheffield-Moore M, Urban RJ, Wolf SE, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84:2705–2711. [DOI] [PubMed] [Google Scholar]
- 20.Wolf SE, Thomas SJ, Dasu MR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendenhall CL, Moritz TE, Roselle GA, et al. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. The VA Cooperative Study Group #275. JPEN J Parenter Enteral Nutr. 1995;19:258–265. [DOI] [PubMed] [Google Scholar]
- 22.Orr R, Fiatarone Singh M. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64:725–750. [DOI] [PubMed] [Google Scholar]
- 23.Dworkin MS, Williamson JM. AIDS wasting syndrome: trends, influence on opportunistic infections, and survival. J Acquir Immune Defic Syndr. 2003;33:267–273. [DOI] [PubMed] [Google Scholar]
- 24.Boughton B. Drug increases lean tissue mass in patients with cancer. Lancet Oncol. 2003;4:135. [DOI] [PubMed] [Google Scholar]
- 25.Church JA. Oxandrolone treatment of childhood hereditary angioedema. Ann Allergy Asthma Immunol. 2004;92:377–378. [DOI] [PubMed] [Google Scholar]
- 26.Stahnke N, Keller E, Landy H. Favorable final height outcome in girls with Ullrich-Turner syndrome treated with low-dose growth hormone together with oxandrolone despite starting treatment after 10 years of age. J Pediatr Endocrinol Metab. 2002;15:129–138. [DOI] [PubMed] [Google Scholar]
- 27.Wolf SE, Edelman LS, Kemalyan N, et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res. 2006;27:131–141. [DOI] [PubMed] [Google Scholar]
- 28.Hart DW, Wolf SE, Zhang XJ, et al. Efficacy of a high-carbohydrate diet in catabolic illness. Crit Care Med. 2001;29:1318–1324. [DOI] [PubMed] [Google Scholar]
- 29.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeschke MG, Chinkes DL, Finnerty CC, et al. Blood transfusions are associated with increased risk for development of sepsis in severely burned pediatric patients. Crit Care Med. 2007;35:579–583. [DOI] [PubMed] [Google Scholar]
- 31.Jeschke MG, Norbury WB, Finnerty CC, et al. Propranolol does not increase inflammation, sepsis, or infectious episodes in severely burned children. J Trauma. 2007;62:676–681. [DOI] [PubMed] [Google Scholar]
- 32.Barrow RE, Mlcak R, Barrow LN, et al. Increased liver weights in severely burned children: comparison of ultrasound and autopsy measurements. Burns. 2004;30:565–568. [DOI] [PubMed] [Google Scholar]
- 33.Rennie MJ. Muscle protein turnover and the wasting due to injury and disease. Br Med Bull. 1985;41:257–264. [DOI] [PubMed] [Google Scholar]
- 34.Herndon DN, Nguyen TT, Wolfe RR, et al. Lipolysis in burned patients is stimulated by the beta 2-receptor for catecholamines. Arch Surg. 1994;129:1301–1305. [DOI] [PubMed] [Google Scholar]
- 35.Demling R, De Santi L. Closure of the “non-healing wound” corresponds with correction of weight loss using the anabolic agent oxandrolone. Ostomy Wound Manage. 1998;44:58–62, 64, 66 passim. [PubMed]
- 36.Demling RH. Oxandrolone, an anabolic steroid, enhances the healing of a cutaneous wound in the rat. Wound Repair Regen. 2000;8:97–102. [DOI] [PubMed] [Google Scholar]
- 37.Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000;15:12–17. [DOI] [PubMed] [Google Scholar]
- 38.Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med. 2000;32:181–186. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe R, Ferrando A, Sheffield-Moore M, et al. Testosterone and muscle protein metabolism. Mayo Clin Proc. 2000;75(suppl):S55–S60. [PubMed] [Google Scholar]
- 40.Zhao J, Bauman WA, Huang R, et al. Oxandrolone blocks glucocorticoid signaling in an androgen receptor-dependent manner. Steroids. 2004;69:357–366. [DOI] [PubMed] [Google Scholar]
- 41.Hasselgren PO. Muscle protein metabolism during sepsis. Biochem Soc Trans. 1995;23:1019–1025. [DOI] [PubMed] [Google Scholar]
- 42.Tiao G, Fagan J, Roegner V, et al. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest. 1996;97:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Luo GJ, Wang JJ, et al. Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock. 1998;10:298–306. [DOI] [PubMed] [Google Scholar]
- 44.Djurhuus CB, Gravholt CH, Nielsen S, et al. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am J Physiol Endocrinol Metab. 2002;283:E172–E177. [DOI] [PubMed] [Google Scholar]
- 45.Krsek M, Rosicka M, Nedvidkova J, et al. Increased lipolysis of subcutaneous abdominal adipose tissue and altered noradrenergic activity in patients with Cushing’s syndrome: an in-vivo microdialysis study. Physiol Res. 2006;55:421–428. [DOI] [PubMed] [Google Scholar]
- 46.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2007;119:e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suman OE, Mlcak RP, Herndon DN. Effect of exercise training on pulmonary function in children with thermal injury. J Burn Care Rehabil. 2002;23:288–293. [DOI] [PubMed] [Google Scholar]
- 48.Suman OE, Spies RJ, Celis MM, et al. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91:1168–1175. [DOI] [PubMed] [Google Scholar]
- 49.Suman OE, Thomas SJ, Wilkins JP, et al. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. J Appl Physiol. 2003;94:2273–2281. [DOI] [PubMed] [Google Scholar]
- 50.Hepatic effects of 17 alpha-alkylated anaboli-androgenic steroids. HIV Hotline. 1998;8:2–5. [PubMed] [Google Scholar]
- 51.Thomas S, Wolf SE, Murphy KD, et al. The long-term effect of oxandrolone on hepatic acute phase proteins in severely burned children. J Trauma. 2004;56:37–44. [DOI] [PubMed] [Google Scholar]
- 52.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med. 2000;28:1578–1584. [DOI] [PubMed] [Google Scholar]
- 53.Schroeder ET, Vallejo AF, Zheng L, et al. Six-week improvements in muscle mass and strength during androgen therapy in older men. J Gerontol A Biol Sci Med Sci. 2005;60:1586–1592. [DOI] [PubMed] [Google Scholar]
- 54.Schroeder ET, Zheng L, Ong MD, et al. Effects of androgen therapy on adipose tissue and metabolism in older men. J Clin Endocrinol Metab. 2004;89:4863–4872. [DOI] [PubMed] [Google Scholar]
- 55.Schroeder ET, Zheng L, Yarasheski KE, et al. Treatment with oxandrolone and the durability of effects in older men. J Appl Physiol. 2004;96:1055–1062. [DOI] [PubMed] [Google Scholar]
- 56.Malhotra A, Poon E, Tse WY, et al. The effects of oxandrolone on the growth hormone and gonadal axes in boys with constitutional delay of growth and puberty. Clin Endocrinol (Oxf). 1993;38:393–398. [DOI] [PubMed] [Google Scholar]