Abstract
Objective:
Pancreatic fistula is a leading cause of morbidity and mortality after pancreaticoduodenectomy. External drainage of pancreatic duct with a stent has been shown to reduce pancreatic fistula rate of pancreaticojejunostomy in a few retrospective or prospective nonrandomized studies, but no randomized controlled trial has been reported thus far. This single-center prospective randomized trial compared the results of pancreaticoduodenectomy with external drainage stent versus no stent for pancreaticojejunal anastomosis.
Methods:
A total of 120 patients undergoing pancreaticoduodenectomy with end-to-side pancreaticojejunal anastomosis were randomized to have either an external stent inserted across the anastomosis to drain the pancreatic duct (n = 60) or no stent (n = 60). Duct-to-mucosa anastomosis was performed in all cases.
Results:
The 2 groups were comparable in demographic data, underlying pathologies, pancreatic consistency, and duct diameter. Stented group had a significantly lower pancreatic fistula rate compared with nonstented group (6.7% vs. 20%, P = 0.032). Radiologic or surgical intervention for pancreatic fistula was required in 1 patient in the stented group and 4 patients in the nonstented group. There were no significant differences in overall morbidity (31.7% vs. 38.3%, P = 0.444) and hospital mortality (1.7% vs. 5%, P = 0.309). Two patients in the nonstented group and none in the stented group died of pancreatic fistula. Hospital stay was significantly shorter in the stented group (mean 17 vs. 23 days, P = 0.039). On multivariate analysis, no stenting and pancreatic duct diameter <3 mm were significant risk factors of pancreatic fistula.
Conclusion:
External drainage of pancreatic duct with a stent reduced leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy.
Previous retrospective and nonrandomized prospective studies suggested that the use of external drainage stent may reduce leakage of pancreaticojejunal anastomosis after pancreaticoduodenectomy. A prospective randomized trial was performed to compare the use of an external drainage stent versus no stent in duct-to-mucosa pancreaticojejunal anastomosis after pancreaticoduodenectomy in 120 patients. This randomized trial showed that stenting of pancreatic duct with an external drainage catheter significantly reduced pancreatic fistula rate.
Pancreaticoduodenectomy is the treatment of choice for patients with resectable carcinoma of the pancreatic head and periampullary region. In recent years, mortality rate of pancreaticoduodenectomy has declined to <5% in many institutions around the world.1–4 However, pancreatic fistula still occurs in 5% to 40% of patients after pancreaticoduodenectomy, depending on the definition of leakage.5–8 The reduction in operative mortality over the past few decades has not been accompanied by a notable improvement of pancreatic fistula rate.9 Hence, it is imperative to conduct research to identify effective strategies to reduce pancreatic leakage after pancreaticoduodenectomy.10
Currently, there is no consensus on the best way of managing the pancreatic stump after pancreaticoduodenectomy. Pancreaticojejunal (PJ) anastomosis is the classic method of reconstruction. Technical modifications such as pancreatic duct occlusion, reinforcement of anastomosis with fibrin glue, placement of internal stent, and pancreaticogastrostomy do not seem to improve the results in prospective randomized trials.10–14 Octreotide has been used to reduce the incidence of pancreatic fistula, but its efficacy remains controversial due to inconsistent results in prospective randomized trials.5,8,15–17 A meta-analysis did not find a benefit of Octreotide in patients undergoing pancreaticoduodenectomy.10 Some retrospective studies have reported low pancreatic fistula rate with the use of a catheter inserted into the pancreatic duct for external drainage of pancreatic jucie.18,19 By diverting away pancreatic juice from the anastomosis, it could theoretically reduce the incidence of PJ anastomotic leakage. A recent prospective but nonrandomized study showed that external drainage of the pancreatic duct decreased the rate of pancreatic fistula from 29.3% to 6.8%, and reduced the median hospital stay from 29 to 13 days.6
Thus far, no prospective randomized trial on the use of external drainage stent for pancreatic anastomosis has been reported. We conducted a prospective randomized trial to test the hypothesis that external drainage of pancreatic duct with a stent could decrease the rate of pancreatic fistula after pancreaticoduodenectomy with PJ anastomosis.
PATIENTS AND METHODS
Between June 2000 and October 2006, 127 patients underwent elective pancreaticoduodenectomy for benign or malignant pathologies of pancreas or periampullary region at Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Hong Kong, China. Of these 127 patients, 120 were recruited into this prospective randomized trial; the other 7 patients refused to participate in the trial. Exclusion criteria included patients undergoing emergency pancreaticoduodenectomy for trauma, and patients with on-going acute pancreatitis at the time of operation. Patients who were recruited before surgery but were found to have unresectable disease after laparoscopy or laparotomy were secondarily excluded from the trial (n = 26). Patients who underwent pancreaticoduodenectomy were randomized by opening consecutive sealed envelopes containing random numbers that assign patients into 2 groups: (1) stented group (n = 60), with an external drainage stent placed across the PJ anastomosis into the pancreatic duct and brought out externally via the jejunal loop and abdominal wall; (2) nonstented group (n = 60), without any stent in the PJ anastomosis. The randomization process (opening of the envelope) was performed during the operation by a nurse not involved in the operative procedure after the resection was completed and immediately before the PJ anastomosis. The study was approved by the Institutional Review Board of our institution, and informed consent was obtained from all patients participating in the trial before surgery. A synopsis of the protocol of the trial has been posted in a clinical trial website of Hong Kong, which is accessible to the public (www.hkclinicaltrials.com; trial number HKCTR-12).
Surgical Techniques
All operations were performed by a team of surgeons specialized in hepatobiliary and pancreatic surgery. Conventional or pylorus-preserving pancreaticoduodenectomy was performed according to the decision of individual surgeon. Lymph nodes around the head of the pancreas, the common hepatic artery, and the hepatoduodenal ligament were dissected. Wedge or segmental resection of the portal vein or superior mesenteric vein was performed when a pancreatic head mass was inseparable from the vein, as described in a previous report from our group.4 For segmental resection of the portal vein or superior mesenteric vein, end-to-end anastomosis without the use of a graft was possible in all cases after adequate mobilization of the portal vein and superior mesenteric vein.
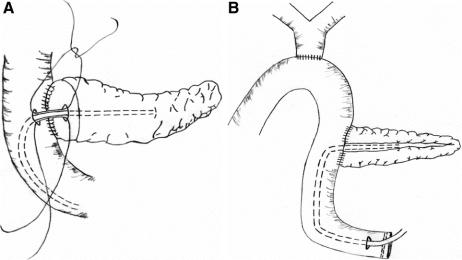
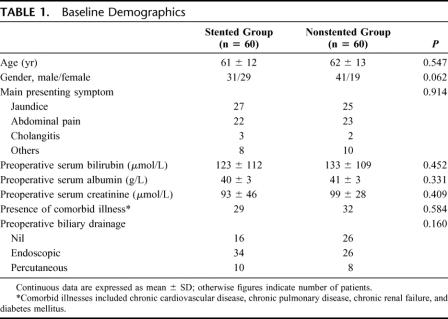
The technique of PJ anastomosis was standardized except for the placement of an external pancreatic duct stent in the stented group. An end-to-side, duct-to-mucosa, 2-layer PJ anastomosis was performed using interrupted fine prolene sutures. The diameter of the pancreatic duct was measured in every case. In the stented group, depending on the size of the pancreatic duct, a Fr 3 - 8 polyvinyl catheter with multiple side-holes was inserted into the pancreatic duct (Fig. 1A). The largest sized stent that could be passed into the pancreatic duct was used. Catheter migration was prevented by an anchoring stitch that secured the catheter onto the mucosa of jejunal side of the PJ anastomosis using a single absorbable suture. Care was taken to ensure that there were no side-holes in the part of the catheter in the jejunum. The catheter exited via a small enterotomy in the free end of the jejunal loop (Fig. 1B), and was externalized through a stab incision in the anterior abdominal wall. The enterotomy site for exit of the catheter was closed with a purse-string suture, and the serosa around the enterotomy site was sutured to the peritoneum of abdominal wall. In the nonstented group, no stent was used in the PJ anastomosis. After PJ anastomosis, an end-to-side, single layer, interrupted hepaticojejunostomy without stenting was performed using the same jejunal loop. A single layer, continuous, hand-sewn antecolic gastrojejunostomy or duodenojejunostomy was then performed, with a nasogastric tube placed into the afferent jejunal limb of the anastomosis. No vagotomy, gastrostomy, or feeding jejunostomy was performed. A drain was placed anterior to the PJ anastomosis, and another 1 posterior to the anastomosis (peripancreatic drains).
FIGURE 1. A, Diagrammatic illustration of end-to-side, duct-to-mucosa pancreaticojejunostomy with an external pancreatic duct stent. The stent was inserted into the pancreatic duct after suturing the back layer of the duct-to-mucosa anastomosis, secured by suturing the catheter to the jejunal mucosa using an absorbable suture, and then the anterior layer of the anastomosis was completed. Only the portion of the catheter within the pancreatic duct had side holes. B, The stent was brought out via an enterotomy in the free end of the jejunal loop, then externalized through a stab incision of the anterior abdominal wall. The enterotomy site was closed with a purse-string suture, and the serosa around the enterotomy site was sutured to the peritoneum on each side to prevent spillage of intestinal content into the peritoneal cavity on removal of the stent.
Perioperative Management
Perioperative management was standardized. All patients received broad-spectrum antibiotics (amoxicillin-clavulanic acid) for 24 hours, and a H2 blocker (famotidine) during the entire postoperative hospital course. No prophylactic somatostatin or Octreotide was used. All patients were managed in the intensive care unit for at least 24 hours. Subsequent need for stay in the intensive care unit was determined according to the patient's condition. The nasogastric tube was removed when bowel sound returned. Patients were kept fasted for the first 5 postoperative days, then oral diet was gradually resumed if there was no evidence of delayed gastric emptying, pancreatic leakage or other intraabdominal complications. Total parenteral nutrition was used only in patients who could not tolerate diet after postoperative day 5.
Drain fluid volume from the peripancreatic drains was measured daily. The serum and drain fluid amylase levels were measured on postoperative day 1, 3, 5, 7, and 9, and then twice a week thereafter if there was evidence of persistent leakage. The peripancreatic drains were removed on postoperative day 10 in both groups if there was no evidence of leakage. If there was evidence of leakage or suspicion of infective complication (fever, leukocytosis, and purulent drain fluid), the peripancreatic drains were left in situ and a contrast computed tomography (CT) scan was performed to look for any intraabdominal collection. In the stented group, the pancreatic duct catheter was connected to a drainage bag during the first 9 days, with daily output measured. If there was no evidence of pancreatic fistula, the catheter was locked and the patient was discharged with the stent in situ until 6 weeks after the operation, when it was removed in the outpatient clinic. If there was evidence of pancreatic fistula, the pancreatic duct catheter was allowed to drain pancreatic juice until the leakage resolved.
Data Collection
Preoperative demographic and clinical data, details of the surgical procedure, pathologic diagnosis, postoperative course and complications were collected prospectively by a research nurse not involved in the care of the patients. Immediately after the operation, the surgeon filled a questionnaire on the type of resection performed, any concomitant procedure, pancreatic texture (soft or firm) and pancreatic duct diameter. The research nurse collected serial data of peripancreatic drain fluid amylase and volume, and the pancreatic drain fluid volume.
Study Endpoints
The primary study end point was pancreatic fistula or leakage, defined as amylase-rich fluid (amylase concentration >3 times the upper limit of normal serum amylase level) collected from the peripancreatic drains after postoperative day 3 with a drainage volume of >10 mL per day according to previous studies.8,15 Pancreatic leakage was further classified into clinical and subclinical leakage.5 Clinical leakage was defined as leakage in association with fever (>38°C), leukocytosis (white cell count >10 × 109/L), sepsis (evidence of infection and hemodynamic instability), need for percutaneous drainage for an amylase-rich fluid collection, or reoperation. Subclinical leakage was defined as a leakage that was asymptomatic and resolved spontaneously with conservative management. Secondary endpoints included overall morbidity rate, hospital mortality rate, duration of stay in intensive care unit, total postoperative hospital stay, number of days to resume oral diet and number of days on total parenteral nutrition.
Sample Size
The sample size was determined assuming a pancreatic fistula rate of 30% in the control group and 10% in the stented group. The pancreatic fistula rate of 30% in the control group was based on previous studies using similar definitions of pancreatic fistula.6,15 The difference in the control group and the stented group was predicted with reference to the result of a previous nonrandomized prospective study.6 To detect a 20% difference in the pancreatic fistula rate with a significance level of 0.05 and statistical power of 80%, a total of 108 patients were required. Assuming that 10% of patients might not complete the study for some reasons, we planned to recruit a total of 120 patients who underwent pancreaticoduodenectomy into the trial before data analysis. Patients who were recruited into the trial but then found to have unresectable disease after laparoscopy or laparotomy were secondarily excluded from the trial and not randomized.
Statistical Analysis
Continuous data were expressed as mean ± SD. Comparisons of categorical and continuous variables were performed using the χ2 test (or Fisher exact test where appropriate) and the unpaired t test, respectively. Multivariate analysis of risk factors for pancreatic fistula was performed using the binary logistic regression analysis. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using a statistical software (SPSS 11.0 for windows, SPSS, Chicago, IL).
RESULTS
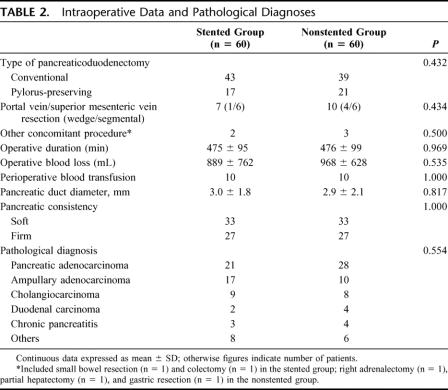
A total of 120 patients who successfully underwent elective pancreaticoduodenectomy were recruited into the trial. Data collection was complete in all patients and there was no protocol violation. Table 1 shows a comparison of baseline demographics of the 2 groups of patients. There were no significant differences between the stented group and nonstented group in gender, age, presenting symptoms, preoperative blood parameters, the presence of comorbid illness, and the proportion of patients with preoperative biliary drainage.
TABLE 1. Baseline Demographics
Table 2 shows the operative data and pathologic diagnoses. The 2 groups were comparable in the proportion of conventional and pylorus-preserving pancreaticoduodenectomy, portal vein resection, other concomitant procedures, operative duration, operative blood loss and transfusion. Pancreatic duct diameter and consistency were also similar between the 2 groups. Carcinoma of the pancreas was the most common pathologic diagnosis in both groups.
TABLE 2. Intraoperative Data and Pathological Diagnoses
Table 3 shows the postoperative outcomes. The pancreatic fistula rate was significantly lower in the stented group compared with the nonstented group (6.7% vs. 20%, P = 0.032). Two of the 4 patients with pancreatic fistula in the stented group had clinical leakage with fever and leukocytosis. One patient required percutaneous drainage of an infected intraabdominal collection. The other patient was treated conservatively with total parenteral nutrition and antibiotics. Nine of the 12 patients with pancreatic fistula in the nonstented group had clinical leakage. Four of these patients had an intraabdominal collection that required percutaneous drainage (n = 2) or reoperation (n = 2). The incidence of clinical pancreatic leakage was significantly lower in the stented group compared with the nonstented group (3.3% vs. 15%, P = 0.027). In the stented group, the mean total volume of pancreatic juice drained from the pancreatic duct catheter over the first 9 days after the operation was 1186 ± 540 mL.
TABLE 3. Postoperative Outcomes
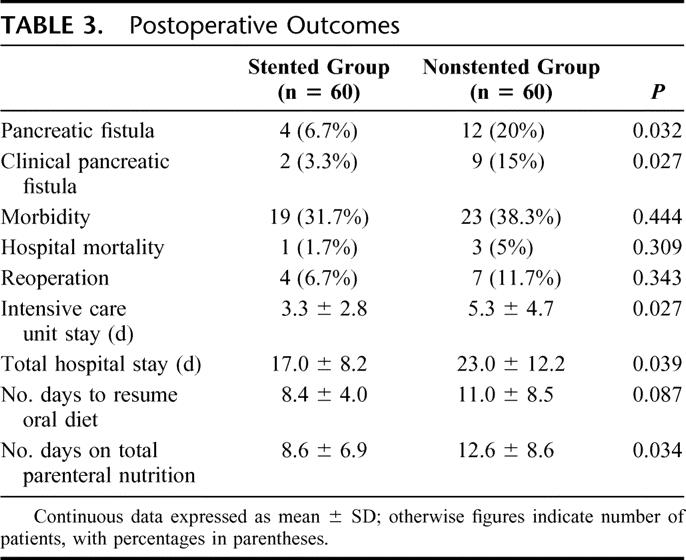
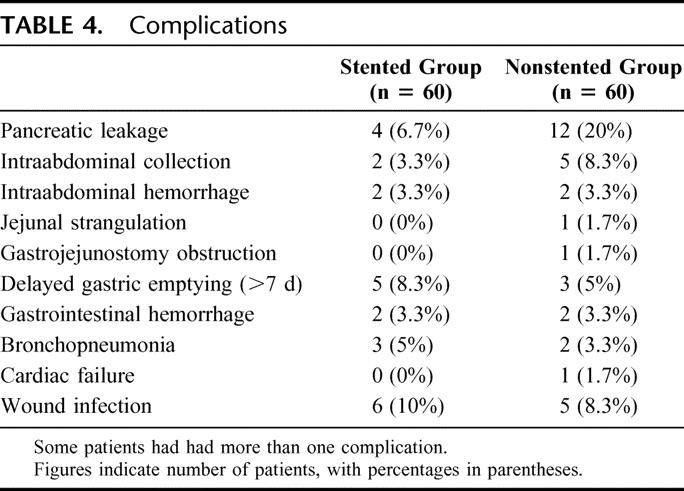
The overall morbidity rate was 31.7% in the stented group and 38.3% in the nonstented group (P = 0.444). Table 4 shows in detail the complications in each group. None of the patients in the stented group had any complication related to the placement or removal of the pancreatic duct stent. Four patients (6.7%) in the stented group and 7 patients (11.7%) in the nonstented group required reoperation, but there was no significant difference in the reoperation rate (P = 0.343). In the stented group, indications for reoperation included intraabdominal hemorrhage (n = 2), intraabdominal collection (n =1) and gastrojejunal anastomosis hemorrhage (n = 1). None of the patients reoperated in the stented group had evidence of pancreatic leakage. Indications for reoperation among the 7 patients in the nonstented group included pancreatic leakage (n = 2), intraabdominal hemorrhage (n = 1), intraabdominal collection (n = 2), gastrojejunostomy obstruction (n = 1) and jejunal strangulation due to adhesion (n = 1). In the 2 patients with leakage of PJ anastomosis who required reoperation, repair of the anastomosis was considered impossible. One patient had the PJ anastomosis converted to pancreaticogastrostomy; the other patient had completion total pancreatectomy.
TABLE 4. Complications
Hospital mortality was lower in the stented group than the nonstented group, but the difference was not significant (1.7% vs. 5%, P = 0.309, Table 3). In the stented group, 1 patient died of severe bronchopneumonia without evidence of pancreatic fistula. In the nonstented group, 4 patients died of postoperative complications. One patient developed pancreatic leakage and died of rupture of common hepatic artery pseudoaneurysm. Another patient died of fungal septicemia from fungus-infected collection resulting from pancreatic leakage. The third patient died of massive bleeding from duodeno-jejunal anastomosis after a pylorus-preserving pancreaticoduodenectomy. The fourth patient died of strangulation of the jejunal limb between hepaticojejunostomy and gastrojejunostomy due to adhesion. This patient was discharged 3 weeks after the pancreaticoduodenectomy but was readmitted 2 weeks later with intestinal obstruction. Hence, the death of this patient was not included as hospital mortality by definition. Overall, 2 patients in the nonstented group and none in the stented group died of pancreatic leakage.
The total postoperative intensive care unit stay was significantly shorter in the stented group than in the nonstented group (Table 3). The stented group also had significantly shorted overall hospital stay (mean 17 vs. 23 days, P = 0.039). There was a trend toward earlier resumption of oral diet in the stented group, but the difference did not reach statistical significance. The duration on total parenteral nutrition was significantly longer in the nonstented group.
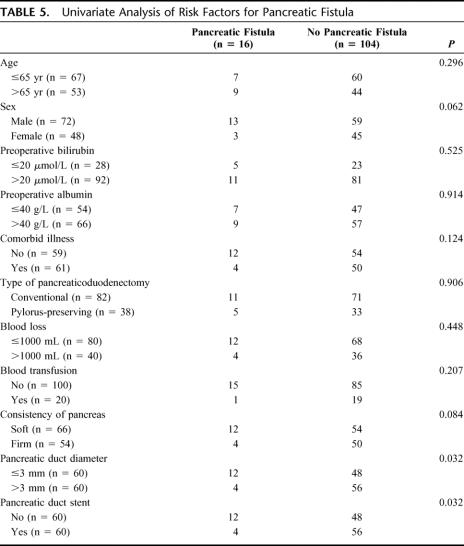
Table 5 shows the results of univariate analysis of factors that may be potentially related to pancreatic fistula. Only 2 factors, pancreatic duct diameter (P = 0.032) and use of pancreatic duct stent (P = 0.032) were significant factors on univariate analysis. There was a trend towards a higher incidence of pancreatic fistula in male patients and in patients with soft pancreatic consistency. A multivariate analysis taking into these 4 factors with P value <0.1 showed that no pancreatic duct stent (risk ratio 3.6496, 95% confidence interval 1.0352–12.8205, P = 0.034) and pancreatic duct diameter ≤3 mm (risk ratio 2.5773, 95% confidence interval 1.0526–9.5238, P = 0.024) were the significant risk factors of pancreatic fistula.
TABLE 5. Univariate Analysis of Risk Factors for Pancreatic Fistula
A further analysis was performed on the pancreatic fistula rate with or without pancreatic duct stent in patients stratified according to pancreatic duct diameter. Among patients with pancreatic duct diameter ≤3 mm, the pancreatic fistula rate was 14.3% (4/28) in the stented group and 25% (8/32) in the nonstented group (P = 0.349). Among patients with pancreatic duct diameter >3 mm, the pancreatic fistula rate was 3.1% (1/32) in the stented group and 10.7% (3/28) in the nonstented group (P = 0.257).
DISCUSSION
Despite substantial improvement in perioperative outcomes of pancreaticoduodenectomy in recent years, pancreatic fistula remains a common complication and the main cause of mortality. To reduce the pancreatic fistula rate, several procedures have been described as alternatives to the conventional PJ anastomosis. Pancreatic duct ligation after pancreaticoduodenectomy was found to be associated with a significantly higher incidence of pancreatic fistula compared with PJ anastomosis.20 Obliteration of the pancreatic duct with fibrin glue or synthetic polymers has been shown to result in a low pancreatic fistula rate of 4% to 7% in retrospective studies.21,22 However, a recent prospective randomized trial showed that duct occlusion without anastomosis did not reduce postoperative complications but significantly increased the risk of endocrine pancreatic insufficiency.11 In fact, the pancreatic fistula rate was significantly higher after duct occlusion compared with PJ anastomosis in that trial. Some retrospective studies have reported lower pancreatic fistula rate with pancreaticogastrostomy instead of PJ anastomosis.23,24 However, 3 prospective randomized controlled trials showed similar pancreatic fistula rates between the 2 types of pancreatic anastomosis,14,25,26 and a recent meta-analysis concluded that the 2 techniques of anastomosis were not different in terms of pancreatic fistula rate and overall morbidity rate.27 PJ anastomosis remains by far the most widely used method of reconstruction for the pancreatic stump after pancreaticoduodenectomy.28 It is obviously important to search for technical improvements to reduce the leakage rate of PJ anastomosis.
In this randomized trial, we tested the hypothesis that external drainage of the pancreatic duct using a stent could reduce the leakage rate of PJ anastomosis after pancreaticoduodenectomy. Internal or external stent across PJ anastomosis is commonly used by many surgeons, but the benefit remains controversial because of scarce data from randomized controlled trials. Theoretically, a stent may help divert away the pancreatic secretion from the anastomosis, and it also allows more precise placement of sutures for duct-to-mucosa anastomosis. Internal stent across PJ anastomosis has been shown to reduce pancreatic leakage in some retrospective studies.29,30 Biehl and Traverso observed a trend towards increased anastomotic integrity and patency with stenting in a randomized study of internal stent versus no stent for duct-to-mucosa PJ anastomosis in a canine model.31 In that study, no anastomotic leakage occurred in the stented group, whereas the leakage rate was 10% without a stent. In contrast, a recent randomized human trial found that the use of a short internal stent did not reduce the frequency or the severity of pancreatic fistula after pancreaticoduodenectomy.13 However, in the latter study, the technique of PJ anastomosis was not standardized as the use of duct-to-mucosa or invagination technique was at the discretion of the surgeons. There could be a possible bias in that invagination technique was chosen for a pancreatic stump with a small pancreatic duct that is more difficult for duct-to-mucosa anastomosis, hence the benefit of a stent could have been missed in such patients.
In the current study, we standardized the technique of PJ anastomosis using end-to-side, duct-to-mucosa anastomosis in all patients to avoid bias of surgeons in selecting the anastomotic technique. A few nonrandomized studies suggested that duct-to-mucosa anastomosis may be associated with a lower leakage rate compared with invagination anastomosis.20,32,33 A prospective randomized study in a canine model has demonstrated that duct-to-mucosa anastomosis was superior to invagination anastomosis in terms of anastomotic patency and function,34 although no randomized human trial has been reported yet. We also standardized other surgical techniques in the operation, except that surgeons were allowed to decide whether to perform a pylorus-preserving or conventional pancreaticoduodenectomy. Prospective randomized trials showed that the 2 types of pancreaticoduodenectomy did not affect pancreatic fistula rate or overall morbidity rate.35 Nonetheless, to eliminate any bias in the choice of the type of pancreaticoduodenectomy in relation to the use of stent, randomization was done only after completion of resection. We did not use prophylactic Octreotide in any patients in view of the lack of clear benefit demonstrated in randomized controlled trials.10
This trial showed that an external pancreatic duct stent significantly reduced leakage rate of PJ anastomosis and hospital stay after pancreaticoduodenectomy. Howard reported no pancreatic leakage in consecutive 56 cases of pancreaticoduodenectomy with PJ anastomosis using an external stent.36 Hamanaka and Suzuki18 reported only 2 cases of minor leakage in 48 patients (4.2%) with PJ anastomosis using an external stent. However, these 2 studies did not include any control group. Subsequently, Roder et al6 reported a prospective but nonrandomized study in 85 patients showing that external drainage of pancreatic duct decreased the pancreatic fistula rate from 29.3% to 6.8% compared with no stent. Our randomized trial showed a similar reduction of pancreatic fistula rate from 20% in the nonstented group to 6.7% in the stented group. It is noteworthy that prophylactic Octreotide was used in the study of Roder et al but not in our study. Of more clinical relevance is the significant reduction of clinical leakage from 15% in the nonstented group to 3.3% in the stented group in the current study. Even when leakage occurred, the severity also appeared to be reduced by the use of a stent. Two patients in the nonstented group had severe PJ leakage requiring conversion to pancreaticogastrostomy and total pancreatectomy, respectively. In contrast, none of the patients with pancreatic fistula in the stented group required reoperation. There were 2 deaths from the pancreatic leakage in the nonstented group but no death from pancreatic leakage in the stented group.
Compared with a short internal stent, an external stent has the theoretical advantage of more complete diversion of pancreatic secretion away from the PJ anastomosis and prevention of activation of pancreatic enzymes by bile. The later may be a particularly important mechanism that explains the benefit of external stent over internal stent. Some surgeons advocated separate Roux-en-Y limbs for the PJ anastomosis and the hepaticojejunostomy to limit pancreatic enzyme activation by bile. A few retrospective series have reported zero pancreatic fistula rate using this technique.37–39 However, this technique has not been compared with single loop reconstruction in prospective trials. Another potential advantage of an external stent over internal stent is that it is associated with a much lower chance of stent passing away from the anastomosis during the first few days after operation when protection of the anastomosis is most required.
The hospital mortality in the stented group was lower than that in the nonstented group (1.7% vs. 5%), but the difference was not statistically significant. The sample size estimation was based on pancreatic fistula rate. A much larger sample size would be required to elucidate whether external drainage stent could reduce postoperative mortality. Similarly, lower morbidity rate and reoperation rate were observed in the stented group but the differences from the nonstented group were not significant. In accordance with the results of other recent studies,13,26,40 the overall morbidity rate was over 30% in this study. In the nonstented group, pancreatic fistula was the most common complication. Given a large enough sample size, stenting may reduce the overall morbidity rate. However, prevention of other complications such as intraabdominal hemorrhage and delayed gastric emptying is also important to reduce the overall morbidity rate.
The pancreatic fistula rate in the nonstented group in this study is comparable to that of 20% to 30% after PJ anastomosis reported in several recent prospective studies.6,12,26,41 In some other studies, lower pancreatic fistula rates of 8% to 11% after PJ anastomosis without stenting have been reported.13,14,17 However, the definitions of pancreatic fistula used in the latter studies were generally more stringent, requiring a higher volume of drain fluid, a higher level of drain fluid amylase level, or a longer duration of persistent drainage of amylase-rich fluid for the diagnosis of pancreatic fistula. The importance of definitions has been recently highlighted in a study, which showed different pancreatic fistula rates in the same group of patients using different definitions.42 In that study of 242 patients with PJ anastomosis after pancreatic resection, the pancreatic fistula rate was 28.5% using a definition of drain output more than 10 mL per day of amylase-rich fluid since the fifth postoperative day, and only 9.9% using a definition of drain output more than 50 mL per day of amylase-rich fluid since the 11th postoperative day. An international study group has recently recommended a definition of pancreatic fistula as drain output of any measurable volume of fluid on or after postoperative day 3 with an amylase content greater than 3 times the serum amylase level, which is similar to our definition in this study.43 The group also concluded that radiologic documentation is neither mandatory nor necessary for diagnosis of pancreatic fistula. In this study, we did not use imaging for diagnosis of pancreatic fistula.
The pancreatic fistula rate of 6.7% in the stented group is almost the same as the pancreatic fistula rate in the stented group in the nonrandomized study by Roder et al.6 Two recent prospective studies also reported low pancreatic fistula rate of 3.6% and 5.4%, respectively, with the use of an external stent for duct-to-mucosa PJ anastomosis.40,44 One of the studies compared internal stent and external stent for PJ anastomosis and reported similar pancreatic fistula rate, but the study was not a randomized trial.44 In that study, the authors observed a higher pancreatic fistula rate with the use of internal stent compared with external stent (14% vs. 8%) in a subgroup of patients with small pancreatic duct, and the authors recommended use of external stent in patients with small pancreatic duct. In the current study, we identified a significantly higher pancreatic fistula rate in patients with small pancreatic duct ≤3 mm compared with patients with wider pancreatic duct. Small pancreatic duct and no stent were the 2 independent risk factors of pancreatic fistula in the multivariate analysis. Two recent studies have also reported pancreatic duct diameter less than 3 mm to be a significant risk factor of pancreatic fistula after pancreaticoduodenectomy.45,46 We performed a subgroup analysis of pancreatic fistula rate according to pancreatic duct diameter. The pancreatic fistula rate was lower with the use of stent compared with no stent in both patients with pancreatic duct diameter ≤3 mm and those with pancreatic duct diameter >3 mm, though the differences were not significant. There was no risk stratification in the initial design of the study and the sample size was probably not adequate for subgroup analysis. A larger sample size study with risk stratification according to pancreatic duct diameter is needed to elucidate whether pancreatic duct drainage may be more beneficial in prevention of anastomotic leakage in patients with small pancreatic duct. Soft pancreatic texture is another risk factor for pancreatic fistula found in some studies.16,47 However, this is a more subjective assessment by the surgeon. In this study, there was a trend of higher fistula rate with soft pancreas, but it was not a significant factor in either univariate or multivariate analysis.
One concern on the use of external drainage stent for PJ anastomosis is the potential complication associated with the stent.13 Ohwada et al44 reported 2 cases of local peritonitis after removal of the stent tube in 37 patients with an external stent after PJ anastomosis. This complication is preventable by careful suturing of the serosa of the jejunal loop around the exit site of the tube to the peritoneum of anterior abdominal wall. We also allowed 6 weeks for formation of adhesions, which further reduced the chance of intestinal content leakage. Similar to other studies on the use of external drainage stent after PJ anastomosis,6,40 no complications related to insertion or removal of the external stent were observed in this study.
In conclusion, this prospective randomized trial showed that the use of an external stent to drain the pancreatic duct significantly reduced the pancreatic fistula rate of duct-to-mucosa PJ anastomosis after pancreaticoduodenectomy.
Discussions
Dr. Keith D. Lillemoe (Baltimore, Maryland): I give the authors particular credit for controlling the many variables involved in the performance of a pancreaticoduodenectomy and its postoperative management. In this study they have shown nicely that external drainage of the pancreatic duct decreases the rate of pancreatic fistula following pancreaticoduodenectomy reconstruction during a Whipple procedure.
Although the authors chose not to formally classify their fistulas by the new International Study Group classification of pancreatic fistulas, they did determine that the incidence of both clinical fistulas represented by fever, leukocytosis, sepsis or need for intervention as well as the self-limited biochemical pancreatic leaks was decreases. Secondary endpoints such as total length of hospital stay, length of ICU stay and the number of days of TPN were also reduced by external drainage. However, their overall mortality, morbidity and incidence of reoperation were not significant different between the 2 groups.
The use of pancreatic stents, including external drainage, is not a new concept and has been used previously by great pancreatic surgeons in the past such as John Howard and William Longmire. This study, however, represents the first Level I evidence to support this practice. And I congratulate the authors for this fine contribution.
I do have a few questions for Dr. Poon. In most institutions, pancreatic fistula is strongly correlated with the texture of the remnant gland. And in your study today it was very close to statistically significant. Although the incident of soft gland was identically distributed between your 2 groups, did you analyze or perform subgroup analysis to determine if external drainage was advantageous to patients with both a soft and a hard gland?
Secondly, how does external stent drainage change your management of an established pancreatic fistula? I see that your mean length of TPN was over 8 days even in the stented group. Are fistulas following external stenting easier to manage and were there differences in the length of stay in those patients with and without a fistula who were either stented not stented?
A few years ago the Hopkins group reported a prospective randomized trial looking at the use of a short non-externally drained stent. That study showed no advantage, can you hypothesize why there was a difference in the outcomes between these 2 studies?
Finally, most groups in the United States have strived over the last decade to reduce the length of stay following pancreaticoduodenectomy. In your study, drains were not routinely removed until day ten, and the total length of stay even in the stented group was in excess of 17 days. I am not attempting to diminish your results, but can you extend your findings to the current practice in the United States where the goal is now to discharge our patients on a diet, drain-free, in less than a week after operation?
Dr. Ronnie T. Poon (Hong Kong, China): For the first question regarding soft and firm pancreas, we performed a subgroup analysis that also showed a reduced leakage rate in patients with soft pancreas among stented patients. The leakage rate was 12% in the stented patients, and 30% in the non-stented patients. For the patient with firm pancreas, the leakage rate was actually similar between the 2 groups. So this may suggest that the stent may be more beneficial in patients who have higher risk of leakage such as those with soft pancreas.
As to your second question, I think our data suggest that the presence of a stent even among patients who have leakage substantially decreases severity of the leakage. If you look at patients who have pancreatic fistula, only 2 of the 4 patients (50%) with fistula in the stented group developed clinical leakage, whereas 9 of the 12 patients in the non-stented group (75%) developed clinical leakage. Furthermore, none of the patients with pancreatic fistula in the stented group required reoperation, whereas 2 patients in the non-stented group required reoperation. And more importantly, there were 2 mortalities from pancreatic fistula in the non-stented group but none in the stented group. So I think the presence of a stent, apart from reducing the incidence of leakage, also reduces severity in case leakage occurs. In the patients who had a stent and developed pancreatic fistula, the fistula could be managed with continued drainage of the stent together with octreotide, and reoperation was not required.
In regards to discrepancies between the study from our group and the previous study by the Johns Hopkins group published in the Journal of Gastrointestinal Surgery in the year 2006, I think there could be 3 possible reasons for the discrepancies.
First, in the study by the Johns Hopkins group, the surgeons were allowed to use both duct-to-mucosa and invagination techniques of pancreaticojejunal anastomosis at their discretion. I think there is a potential for a selection bias in that surgeons might use invagination anastomosis in patients who had small pancreatic duct size, which is more difficult for duct-to-mucosa anastomosis. This may reduce the benefit of stenting, because stenting would probably benefit more those patients with small pancreatic duct, especially with regard to allowing better placement of sutures.
Second, the long external stent could decrease the chance of stent migration, whereas with the use of a short internal stent, there is a possibility that the stent might have migrated away from the anastomosis in the first few post-operative days when protection of the anastomosis was critical. You can never be sure whether the stent is still there with the use of an internal stent. With the use of an external stent, from the pancreatic juice drainage, you know that the stent is still in place.
Third, and probably the most important reason, is that external stents offer better diversion of the pancreatic juice away from the anastomosis. A short internal stent only diverts juice to the jejunal side in vicinity to the anastomosis, and I think this may not be enough to protect the anastomosis from leakage. Also, an external stent prevents activation of the pancreatic enzymes bile, which a lot of surgeons believe is an important element in pancreatic leakage.
Finally, regarding the question on the hospital stay, there is obviously a difference in surgical practice between Hong Kong and the United States. In Hong Kong, most patients would not leave the hospital until they completely recover because they do not have to pay for the hospital stay. The government pays for the hospital stay. In the United States, the patients or Medicare pay for the hospital stay. So I think the benefit in terms of hospital stay may not be as dramatic in the United States, although I would still expect that with reduced clinical leakage there will be a benefit in the overall reduction in terms of hospital stay even in the United States.
Dr. L. William Traverso (Seattle, Washington): I have been looking for a study just like this, not for the reasons that you present but to understand the physiology that happens after a Whipple that promotes delayed gastric emptying. Some people see this phenomenon frequently. Some people see it less. But your study offers some clues, and I want to ask you 1 question.
Dr. Zollinger in the 1950s, through many studies in his laboratory, showed that excluding pancreatic juice from the intestine immediately resulted in an increase in gastric acid secretion and an increase in gastric volume. Therefore, I would like to ask you, by excluding the pancreatic juice from the intestine as best the stent could do, did you see an increase in nasogastric tube volumes per day in the stent compared to the control group? Our idea is that an internal stent prevents this increased gastric volume and acid in the perioperative period at a time when the newly reconstructed GI tract of the patient cannot handle this as well as an increased load of intestinal juice.
Dr. Ronnie T. Poon (Hong Kong, China): I think that is an interesting hypothesis. We did measure the output from the nasogastric tubes and documented it in the database. I also ran an analysis of nasogastric tube output, and there was no difference between the 2 groups. There was no difference in the output or the incidence of delayed gastric emptying. So our data did not seem to support that hypothesis. But I think that is a very interesting physiological hypothesis.
Dr. Steven M. Strasberg (St. Louis, Missouri): Dr Poon, you actually presented 2 types of pancreatic fistula, namely clinically relevant pancreatic fistulas and fistulas that were not clinically relevant. The latter type of fistula, for instance 1 with 10 milliliters of drainage of high amylase fluid on postoperative day 5 but with no other abnormalities and without change in management, would not even fit the definition of a surgical complication. In fact, that is a type A fistula in the classification promoted by the ISGPF. Clinically relevant fistulas are obviously what we want to record if we are interested in complications.
Also, when there is failure at the pancreatic anastomosis, this may result in either fistula or intraabdominal abscess. Were there abscesses and was the incidence the same in the 2 groups?
Dr. Ronnie T. Poon (Hong Kong, China): We only performed CT scans when there was evidence of sepsis, fever or leukocytosis. So we did not have the incidence of collections in all patients. But from our data, among the patients with CT scans, there were 2 collections in the stented group and 5 in the non-stented group. One collection in the stented group and 4 collections in the non-stented group turned out to be infected by either blood culture or culture of aspiration of the collection. So these data suggest that in the non-stented group, there was a higher incidence of abscess. But I think the data were not conclusive.
Dr. Daniel T. Dempsey (Philadelphia, Pennsylvania): I may have missed it, but I did not see the data on how much your stents drained. I used 5 French pediatric feeding tubes as stents for about 20 years. And the residents love to point out to me that about a quarter of the time they do not drain much. So that is my first question, was there a big variability in how much your patients drain?
The second question, unless you control for how these patients are managed when the antibiotics are stopped, when they leave the ICU, when you start to feed them, when you stop the TPN, isn't it possible that the lower fistula rate is due to the fact that they are managed differently simply because they got a piece of plastic hanging out of their abdomen rather than what the plastic tube is supposed to be doing?
Dr. Ronnie T. Poon (Hong Kong, China): In response to your first question, we used polyvinyl catheters of a size ranging from 3 to 8 French, and we chose the catheter with the largest size that could fit into the pancreatic ducts. We actually measured pancreatic duct drain fluid volume daily. From our data, the average drain volume over the first 9 days was around 1200 mLs. Of course, there are variations among patients. Sometimes even with a small duct, we did observe significant output from the pancreatic duct drain.
And for your second question, research nurses collected the data independent of the clinicians managing the patients, and the diagnosis of pancreatic fistula was based purely on data collected regarding the volume and amylase levels in the peri-pancreatic drains. Hence, I think there is little chance of bias due to management by the clinicians.
Footnotes
Reprints: Ronnie T. Poon, MS, PhD, FRCS (Edin), FACS, Department of Surgery, Queen Mary Hospital, The University of Hong Kong, 102 Pokfulam Road, Hong Kong, China. E-mail: poontp@hkucc.hku.hk.
REFERENCES
- 1.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130:295–300. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman MD, Kilburn H, Lindsey M, et al. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s. Ann Surg. 1997;3:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Lo CM, et al. Pancreaticoduodenectomy with en bloc portal vein resection for pancreatic carcinoma with suspected portal vein involvement. World J Surg. 2004;28:602–608. [DOI] [PubMed] [Google Scholar]
- 5.Lowy AM, Lee JE, Pisters PW, et al. Prospective randomized trial of Octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg. 1997;226:632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roder JD, Stein HJ, Bottcher KA, et al. Stented versus nonstented pancreaticojejunostomy after pancreatoduodenectomy. A prospective study. Ann Surg. 1999;229:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montorsi M, Zago M, Mosca F, et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resection. A prospective, controlled, randomized clinical trial. Surgery. 1995;117:26–31. [DOI] [PubMed] [Google Scholar]
- 9.Strasberg SM, Drebin JA, Soper NJ. Evolution and current status of the Whipple procedure: update for gastroenterologists. Gastroenterology. 1997;113:983–994. [DOI] [PubMed] [Google Scholar]
- 10.Poon RT, Lo SH, Fong D, et al. Prevention of pancreatic anastomotic leakage after pancreaticoduodenectomy. Am J Surg. 2002;183:42–52. [DOI] [PubMed] [Google Scholar]
- 11.Tran K, Van Eijck C, Di Carlo V, et al. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg. 2002;236:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lillemoe KD, Cameron JL, Kim MP, et al. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2004;8:766–772. [DOI] [PubMed] [Google Scholar]
- 13.Winter JM, Cameron JL, Campbell KA, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10:1280–1290. [DOI] [PubMed] [Google Scholar]
- 14.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992;163:125–130. [DOI] [PubMed] [Google Scholar]
- 16.Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000;232:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesse UJ, DeDecker C, Houtmeyers P, et al. Prospectively randomized trial using perioperative low dose octreotide to prevent organ-related and general complications after pancreatic surgery and pancreatico-jejunostomy. World J Surg. 2005;29:1325–1328. [DOI] [PubMed] [Google Scholar]
- 18.Hamanaka Y, Suzuki T. Total pancreatic duct drainage for leakproof pancreatojejunostomy. Surgery. 1994;115:22–26. [PubMed] [Google Scholar]
- 19.Mok KT, Wong BW, Liu SI. Management of pancreatic remnant with strategies according to the size of pancreatic duct after pancreaticoduodenectomy. Br J Surg. 1999;86:1018–1019. [DOI] [PubMed] [Google Scholar]
- 20.Bartoli FG, Arnone GB, Ravera G, et al. Pancreatic fistula and relative mortality in malignant disease after pancreaticoduodenectomy. Review and statistical meta-analysis regarding 15 years of literature. Anticancer Res. 1991;11:1831–1848. [PubMed] [Google Scholar]
- 21.Marczell P, Stierer M. Partial pancreaticoduodenectomy (Whipple procedure) for pancreatic malignancy: occlusion of a non-anastomosed pancreatic stump with fibrin sealant. HPB Surg. 1992;5:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Carlo V, Chiesa R, Pontiroli AE, et al. Pancreaticoduodenectomy with occlusion of the residual stump with Neoprene injection. World J Surg. 1989;13:105–111. [DOI] [PubMed] [Google Scholar]
- 23.Miyagawa S, Makuuchi M, Lygidakis NJ, et al. A retrospective comparative study of reconstructive methods following pancreaticoduodenectomy—pancreaticojejunostomy versus pancreaticogastrostomy. Hepatogastroenterology. 1992;39:381–384. [PubMed] [Google Scholar]
- 24.Kim SW, Youk EG, Park YH. Comparison of pancreatogastrostomy and pancreatojejunostomy after pancreatoduodenectomy performed by one surgeon. World J Surg. 1997;21:640–643. [DOI] [PubMed] [Google Scholar]
- 25.Bassi C, Falconi M, Molinari E, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242:767–771; discussion 771–773. [DOI] [PMC free article] [PubMed]
- 26.Duffas JP, Suc B, Msika S, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189:720–729. [DOI] [PubMed] [Google Scholar]
- 27.Wente MN, Shrikhande SV, Muller MW, et al. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg. 2007;193:171–183. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Usui S, Kajiwara H, et al. Current pancreatogastrointestinal anastomosis methods: results of a Japanese survey of 3109 patients. J Hepatobiliary Pancreat Surg. 2004;11:25–33. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimi F, Ono H, Asato Y, et al. Internal stenting of the hepaticojejunostomy and pancreaticojejunostomy in patients undergoing pancreatoduodenectomy to promote earlier discharge form hospital. Surg Today. 1996;26:665–667. [DOI] [PubMed] [Google Scholar]
- 30.Shibuya T, Uchiyama K, Imai S, et al. Improvement of pancreaticojejunostomy in pancreatoduodenectomy. Int Surg. 1995;80:57–60. [PubMed] [Google Scholar]
- 31.Biehl T, Traverso LW. Is stenting necessary for a successful pancreatic anastomosis? Am J Surg. 1992;63:530–532. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto Y, Fujii H, Miura K, et al. Successful pancreatojejunal anastomosis for pancreatoduodenectomy. Surg Gynecol Obstet. 1992;175:555–562. [PubMed] [Google Scholar]
- 33.Hosotani R, Doi R, Imamura M. Duct-to-mucosa pancreaticojejunostomy reduces the risk of pancreatic leakage after pancreatoduodenectomy. World J Surg. 2002;26:99–104. [DOI] [PubMed] [Google Scholar]
- 34.Greene S, Loubeau JM, Peoples JB, et al. Are pancreatoenteric anastomoses improved by duct-to-mucosa sutures? Am J Surg. 1991;161:45–50. [DOI] [PubMed] [Google Scholar]
- 35.Diener MK, Knaebel HP, Heukaufer C, et al. A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg. 2007;245:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard JM. Pancreatojejunostomy: leakage is a preventable complication of the Whipple operation. J Am Coll Surg. 1997;184:454–457. [PubMed] [Google Scholar]
- 37.Kingsnorth AN. Safety and function of isolated Roux loop pancreaticojejunostomy after Whipple's pancreaticoduodenectomy. Ann R Coll Surg Engl. 1994;76:175–179. [PMC free article] [PubMed] [Google Scholar]
- 38.Papadimitriou JD, Fotopoulos AC, Smyrnitotis B, et al. Subtotal pancreatoduodenectomy: use of a defunctionalized loop for pancreatic stump drainage. Arch Surg. 1999;134:135–139. [DOI] [PubMed] [Google Scholar]
- 39.Khan AW, Agarwal AK, Davidson BR. Isolated Roux Loop duct-to-mucosa pancreaticojejunostomy avoids pancreatic leaks in pancreaticoduodenectomy. Dig Surg. 2002;19:199–204. [DOI] [PubMed] [Google Scholar]
- 40.Langrehr JM, Bahra M, Jacob D, et al. Prospective randomized comparison between a new mattress technique and Catell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg. 2005;29:1111–1119. [DOI] [PubMed] [Google Scholar]
- 41.Gouillat C, Chipponi J, Baulieux J, et al. Randomized controlled multicentre trial of somatostatin infusion after pancreaticoduodenectomy. Br J Surg. 2001;88:1456–1462. [DOI] [PubMed] [Google Scholar]
- 42.Bassi C, Butturini G, Molinari E, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Digest Surg. 2004;21:54–59. [DOI] [PubMed] [Google Scholar]
- 43.Bassi C, Dervanis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 44.Ohwada S, Tanahashi Y, Ogawa T, et al. In situ vs ex situ pancreatic duct stents of duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy with billroth I-type reconstruction. Arch Surg. 2002;137:1289–1293. [DOI] [PubMed] [Google Scholar]
- 45.Yang YM, Tian XD, Zhuang Y, et al. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muscari F, Suc B, Kirzin S, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006;139:591–598. [DOI] [PubMed] [Google Scholar]
- 47.Lin JW, Cameron JL, Yeo CJ, et al. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–959. [DOI] [PubMed] [Google Scholar]