Abstract
Objectives:
To describe the effect of the splenic allograft in human multivisceral transplantation.
Summary Background Data:
We performed transplants of the spleen as part of a multivisceral graft in an attempt to decrease both the risk of infection from an asplenic state and the risk of rejection by a possible tolerogenic effect. To our knowledge, this is the first report of human splenic transplantation in a large series.
Methods:
All primary multivisceral recipients who received a donor spleen (N = 60) were compared with those who did not receive a spleen (N = 81).
Results:
Thirty-five of 60 (58%) are alive in the spleen group, and 39 of 81 (48%) are alive in control group (P = 0.98). In univariate analysis, splenic recipients showed superiority in freedom-from-any rejection (P = 0.02) and freedom-from-moderate or severe rejection (P = 0.007). No significant differences were observed in analyses of infectious complications between the spleen and control groups. Both platelet and leukocyte counts became normal in splenic patients, whereas these counts were significantly increased in nonsplenic recipients. Observed incidence of graft versus host disease (GVHD) was 8.25% (5 of 60) in the spleen group and 6.2% (5 of 81) in the control group (P = 0.70). Increased incidence of autoimmune hemolysis was observed in the spleen group.
Conclusions:
Allograft spleen can be transplanted within a multivisceral graft without significantly increasing the risk of GVHD. The allogenic spleen seems to show a protective effect on small bowel rejection. Further investigation with longitudinal follow-up is required to precisely determine the immunologic and hematologic effects of the allograft spleen.
We transplanted a human spleen en bloc with a multivisceral graft in 60 patients. Allograft spleen showed some evidence of a lower rejection rate and no significantly increased risk of graft versus host disease was observed.
The spleen is the largest single secondary lymphoid organ and a vital site of the reticuloendothelial system. As such, this organ plays a major role in both adaptive and innate immune responses. Healthy individuals that have undergone posttraumatic splenectomy have long-term impairment of humoral and cellular immunity.1 Specifically, these patients are extremely susceptible to encapsulated bacteria infection from Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B and similar organisms.1 The polysaccharide capsules of these bacteria elicit a T-cell independent immune response that depends on the function of the spleen’s marginal B cells.2 Splenectomized patients have diminished responses to such antigens.3 In children less than 5 years of age, the risk of overwhelming postsplenectomy sepsis may be increased 60- to 100-fold compared with children who have not had a splenectomy.4
Multivisceral transplantation (MVT) has been successfully performed in adults and children with intestinal and liver failure,5,6 with the results having improved dramatically in recent years.5,6 The traditional procedure for MVT is to transplant the stomach, pancreas, intestine, and liver en bloc. The spleen of the recipient is removed during the procedure, leaving MVT recipients in an asplenic state. Because of the concern of an increased risk of sepsis with an asplenic state, we have included the allograft spleen as part of the multivisceral graft since 2001. In animal models, donor splenic transplantation is known to induce donor-specific tolerance.7–13 Sporadic cases of splenic transplantation in humans have been reported in the past,8–27 as part of a pancreas transplant or in an attempt to treat hematological disorders. No previous literature is available on the effect of human splenic allograft for tolerogenicity. In this manuscript, we evaluate and compare our experience of multivisceral transplant recipients who did and did not receive a splenic allograft to elucidate the effect of this donor-derived lymphoid organ in human transplantation.
PATIENTS AND METHODS
All patients who received a primary multivisceral transplant with the inclusion of the spleen allograft at our institution were studied (spleen group). Their outcomes were compared with all primary multivisceral recipients who did not receive a spleen at our institution since the inception of the program (control group).
Standard multivisceral transplantation (MV) refers to an en bloc transplant of the stomach, pancreas, liver, and intestine. Modified multivisceral transplant (MMV) refers to MVT without the liver (ie, en bloc transplant of the stomach, pancreas, and intestine). Since both MV and MMV patients received spleen as part of the allograft, they were both included in this paper. To accurately assess the impact of allograft spleen, only children who received MV were compared in some of the analyses in addition to the comparison in the entire cohort.
The surgical technique, immunosuppressive protocols, and postoperative management of our patients are described elsewhere.28–30 Maintenance immunosuppression included tacrolimus and corticosteroids except for those patients who received alemtuzumab (Campath, Genzyme, Cambridge, MA) induction, in whom corticosteroids were avoided. Patients who received daclizumab (Zenapax, Roche Laboratories, Nutley, NJ) as induction immunosuppressive therapy were given 2 mg/kg intravenous administration at surgery followed by 2 mg/kg at days 4, 7 and once every 2 weeks thereafter during the first 3 months, reduced to 1 mg/kg once every 2 weeks during the following 3 months posttransplant. Patients who received alemtuzumab as induction were given 0.3 mg/kg on the day of surgery, and on postoperative days 4 and 7 (3 doses total); subsequently, a small subset of patients received only 2 doses of Campath 1H, on day 0 and day 4 posttransplant. Small bowel graft rejection was monitored with protocol endoscopy and biopsy according to the following schedule: twice a week during the first month, then once a week during the next 2 months, and then once every 2 to 4 weeks until stoma closure. This study was performed under the protocols approved by our institutional review board. An appropriate informed consent was obtained from the patients (or the parents).
Mixed Lymphocyte Reaction (MLR)
The proliferative responses of recipients were monitored using a standard 3H-thymidine incorporation assay.31 The stimulation indices (S.I.) were calculated using the formula
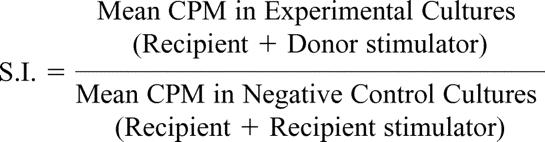 |
Statistical Analysis
Multivariate Cox stepwise regression analysis was performed for the hazard rate of developing any rejection and additionally for moderate or severe rejection. Similar regressions for the incident rates of various infectious episodes were analyzed as well as for graft survival.
RESULTS
Baseline Characteristics
A total of 60 patients received spleen as part of the multivisceral graft (spleen group), while 81 patients received traditional multivisceral grafts (control group). Distributions of the baseline characteristics of patients between the spleen and control groups are summarized in Table 1. Fifty-one patients in the spleen group were recipients of a MV, and 9 received a MMV. Sixty-eight patients in the control group received a MV, and 13 received a MMV.
TABLE 1. Distributions of the Baseline Characteristics of Patients Between the Spleen and Control Groups
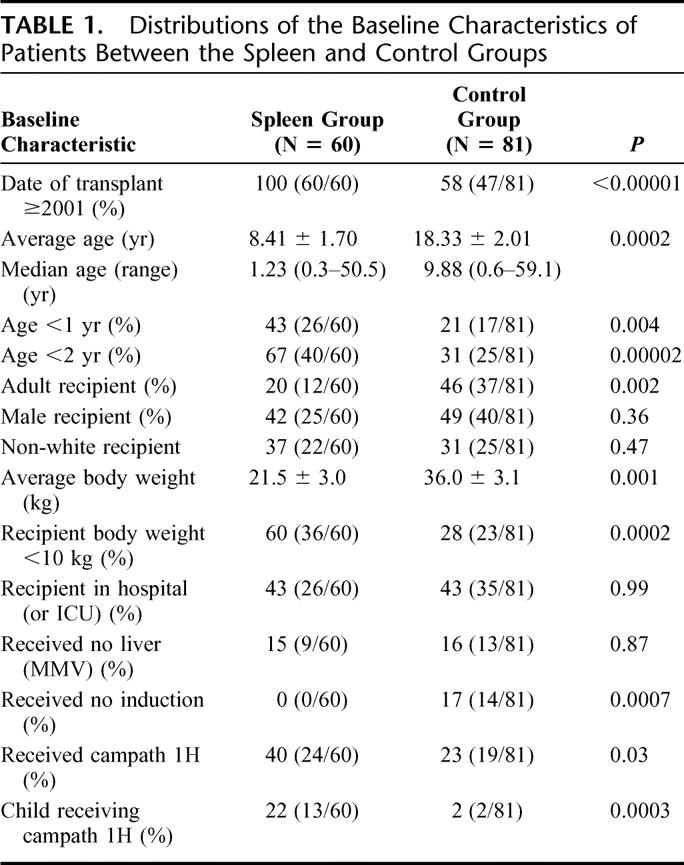
Splenic recipients underwent transplantation between October 2001 and February 2007; control patients underwent transplantation between December 1994 and February 2007, with 34 of these patients undergoing transplantation before year 2001. Thus, splenic recipients were significantly more likely to undergo transplantation since 2001 (100% vs. 58%, P < 0.00001).
There were 49 adults and 92 children in the entire cohort, and children were significantly more likely to have received a spleen, with the percentage being 52.2% (48 of 92) in children versus 24.5% (12 of 49) in adults (P = 0.002). Median ages at transplant were 30.6 and 1.0 years among adults and children in the spleen group, and 33.4 and 1.3 among those in the control group, respectively. The percentage of patients whose age was less than 2 years was significantly increased in the spleen group, 67% (40 of 60) versus 31% (25 of 81) in the control group (P = 0.00002). The percentage of patients whose age was less than 1 year was also significantly increased in the spleen group (Table 1).
All patients (100%) in the spleen group received induction immunosuppression, whereas 14 (17%) in the control group did not (P = 0.0007). Patients in the spleen group were more likely to receive Campath 1H induction (40% vs. 23%, P = 0.03), and especially more children received Campath 1H induction in the spleen versus control group (22% vs. 2%, P = 0.003).
Patient and Graft Survival
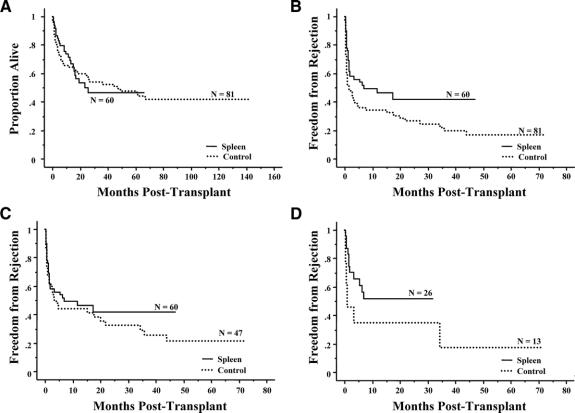
A total of 74 (35 spleen, 39 control) patients were still alive in the entire cohort as of the last follow-up date, (March 1, 2007). The Kaplan-Meier curves in Figure 1A display no statistical difference in patient survival between the spleen and control groups (P = 0.98). The 2 longest survivors in the spleen group have now lived more than 5 years, with currently stable graft function.
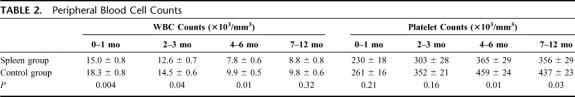
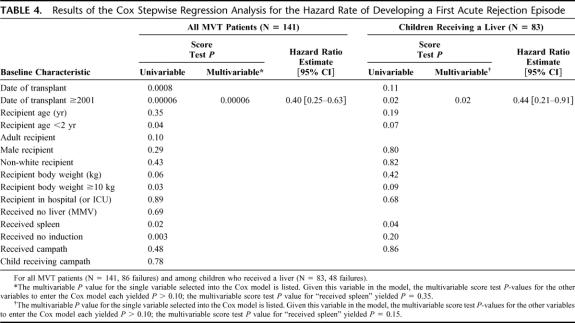
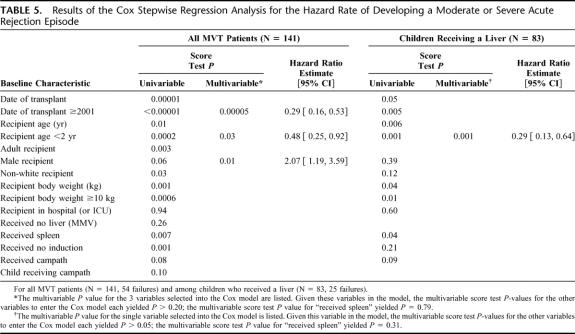
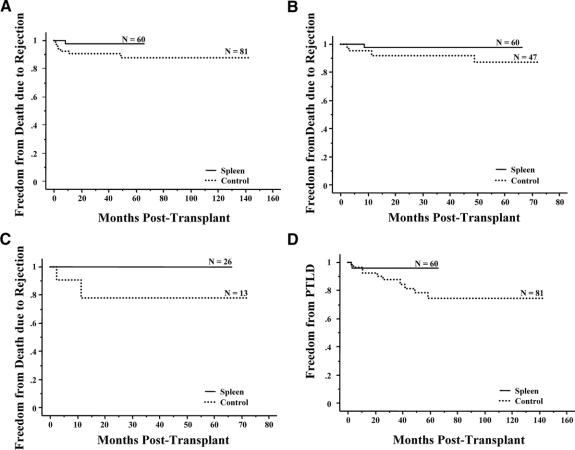
FIGURE 1. A, Kaplan-Meier comparison of patient survival between patients who received (N = 60, 25 deaths) versus did not receive a spleen (N = 81, 42 deaths) (P = 0.98). B, Kaplan-Meier freedom-from-rejection comparison between patients who received (N = 60, 29 failures) versus did not receive a spleen (N = 81, 57 failures) (P = 0.02). C, Kaplan-Meier freedom-from-rejection comparison of patients transplanted since 2001 between those who received (N = 60, 29 failures) versus did not receive a spleen (N = 47, 31 failures) (P = 0.39). D, Kaplan-Meier freedom-from-rejection comparison of children less than 1 year of age who underwent transplantation since 2001 and received (N = 26, 12 failures) versus did not receive a spleen (N = 13, 9 failures) (P = 0.07).
Prognostic factors for graft survival were analyzed in the overall cohort. Cox regression analysis revealed 2 factors to be significant: date of transplant ≥2001 (favorable, P = 0.004) and child receiving Campath-1H (unfavorable, P = 0.0003). Receiving spleen did not affect graft survival (P = 0.95).
Peripheral Blood Cell Counts
Peripheral white blood cell (WBC) counts and platelet counts were compared between the spleen and control groups (Table 2). Average WBC counts (×103/mm3) at 0 to 1 month, 2 to 3 months, 4 to 6 months, and 7 to 12 months posttransplant were 15.0 ± 0.8, 12.6 ± 0.7, 7.8 ± 0.6, and 8.8 ± 0.8 in the spleen group and 18.3 ± 0.8, 14.5 ± 0.6, 9.9 ± 0.5, and 9.8 ± 0.6 in the control group, respectively. Average WBC counts were significantly increased in the control group at 0 to 1 month (P = 0.004), 2 to 3 months (P = 0.04), and 4 to 6 months (P = 0.01) but was not different at 7 to 12 months (P = 0.32). Average platelet counts (×103/mm3) at 0 to 1 month, 2 to 3 months, 4 to 6 months and 7 to 12 months posttransplant were 230 ± 18, 303 ± 28, 365 ± 29, and 356 ± 29 in the spleen group and 261 ± 16, 352 ± 21, 459 ± 24, and 437 ± 23 in the control group, respectively. In contrast to the WBC counts, the average platelet count was significantly increased in the control group at 4 to 6 months (P = 0.01) and 7 to 12 months (P = 0.03) but was not different at 0 to 1 month (P = 0.21) and 2 to 3 months (P = 0.16) posttransplant.
TABLE 2. Peripheral Blood Cell Counts
Rejection
Average number of observed episodes of acute cellular rejection was 1.08 ± 0.21 per patient in the spleen group and 1.23 ± 0.15 per patient in control group (P = 0.55). The average incidence of acute rejection was 0.34 ± 0.07 per 100 patient-days in the spleen group and 0.57 ± 0.12 per 100 patient-days in the control group (P = 0.10).
Freedom from rejection curves are displayed in Figure 1B, and in comparing the spleen versus control groups, splenic recipients showed significant superiority in freedom from rejection (P = 0.02). Although differences were no longer significant, there was a trend towards less rejection in the spleen group after eliminating the patients who were transplanted before 2001 (Fig. 1C). This trend was more apparent in patients less than 1 year of age who were transplanted since 2001 (Fig. 1D).
Prognostic factor analysis showed 5 factors to be favorably associated with freedom from rejection in univariable analysis: date of transplant ≥2001 (P = 0.00006), age <2 years (P = 0.04), recipient weight <10 kg (P = 0.03), receiving spleen (P = 0.02), and receiving induction (P = 0.003) (Table 4). Among those factors, Cox stepwise (multivariable) regression analysis showed that only date of transplant ≥2001 was independently significant. However, there was a strong correlation between date of transplant ≥2001 and receiving a spleen (Table 1). These results were consistent with those obtained when the analysis was performed in children receiving a liver (pediatric MV recipients) (Table 4).
TABLE 4. Results of the Cox Stepwise Regression Analysis for the Hazard Rate of Developing a First Acute Rejection Episode
Similar prognostic factor results regarding the impact of splenic inclusion were obtained for freedom from the development of a moderate or severe rejection (Table 5). Although the inclusion of spleen was a significant factor favorably associated with freedom from moderate or severe rejection in univariate analysis, it was not significant in multivariate analysis (date of transplant ≥2001, recipient age <2 and male recipient were significant in the entire cohort, and only recipient age <2 was significant in the cohort of pediatric MV recipients. Again, there were strong correlations among date of transplant ≥2001, recipient age <2, and receiving a spleen (Table 1).
TABLE 5. Results of the Cox Stepwise Regression Analysis for the Hazard Rate of Developing a Moderate or Severe Acute Rejection Episode
In the spleen group, only one patient graft loss was attributed to rejection. In the control group, graft loss due to rejection was observed in 7 patients. Figures 2A–C compare freedom from graft failure or death (graft loss) due to rejection between the spleen and no spleen groups for all patients, those transplanted since 2001, and in children less than 1 year of age who were transplanted since 2001, respectively. Analysis of children less than 1 who were transplanted since 2001 revealed that patients in the spleen group showed significantly improved freedom from graft loss due to rejection (P = 0.03) and trends towards superiority of splenic recipients were observed in the entire cohort (P = 0.10) and in the subset of patients transplanted since 2001 (P = 0.21).
FIGURE 2. A, Kaplan-Meier freedom-from-graft failure or death due to rejection comparison between patients who received (N = 60, 1 failure) versus did not receive a spleen (N = 81, 7 failures) (P = 0.10). B, Kaplan-Meier freedom-from-graft failure or death due to rejection comparison of patients underwent transplantation since 2001 between those who received (N = 60, 1 failure) versus did not receive a spleen (N = 47, 4 failures) (P = 0.21). C, Kaplan-Meier freedom-from-graft failure or death due to rejection comparison of children <1 year of age who underwent transplantation since 2001 and received (N = 26, 0 failures) versus did not receive a spleen (N = 13, 2 failures) (P = 0.03). D, Kaplan-Meier freedom-from-development of post-transplant lymphoproliferative disease comparison between patients who received (N = 60, 2 failures) versus did not receive a spleen (N = 81, 10 failures) (P = 0.26).
Infection
Average number of observed episodes of infection (including bacterial, fungal, and viral infection) ±1 standard error was 4.77 ± 0.51 in the spleen group and 5.96 ± 0.51 in the control group (P = 0.11). Incidence rates of infection were 2.46 ± 0.46 per 100 patient-days in the spleen group and 3.88 ± 0.89 per 100 patient-days in the control group (P = 0.16). Although there was a trend for a lower incidence of infection in the spleen group, the differences were not statistically significant. The same trend was observed for infections that occurred within the first 3 months posttransplant (average number of episodes was 3.02 ± 0.28 vs. 3.72 ± 0.31 in the spleen vs. control groups).
Fungal infections (invasive and noninvasive) were observed in 20 patients (33%) in the spleen group and in 34 patients (42%) in the control group. No differences between the spleen and control groups were observed in freedom from fungal infection.
In the spleen group, infection was the cause of death in 11 patients (death due to infection). In the control group, death due to infection was observed in 17 patients. There was no significant difference in the infection death rate between the 2 groups.
Graft Versus Host Disease (GVHD)
Observed incidence of GVHD was 8.25% (5 of 60) in the spleen group and 6.2% (5 of 81) in the control group (P = 0.70, log-rank test); observed mortality due to GVHD was 1.7% (1 of 60) in the spleen group and 2.5% (2 of 81) in the control group. One child in the spleen group who underwent donor splenectomy as treatment for the GVHD improved without further therapy and is currently doing well without recurrence more than 2 years from the resolution of the initial rash. Interestingly, his level of chimeric donor cells did not change during and after GVHD (0.049% during GVHD and 0.061% immediately after the resolution). An additional patient in the spleen group experienced GVHD resolution without need for splenectomy. The single fatal case in the spleen group developed GVHD at 140 days after transplant. He received a first dose of Campath 1H to treat the disease, with immediate response. He then developed a recurrent rash 4 months after the first infusion and received another treatment dose of Campath 1H with again immediate resolution. However, this patient went on to develop recurrent episodes of infection with a multidrug resistant-organism and died of sepsis 5 months later.
Posttransplant Lymphoproliferative Disease (PTLD)
Observed incidence of PTLD was 3.3% (2 of 60) in the spleen group and 12.3% (10 of 81) in the control group. Freedom from PTLD shows a trend towards less PTLD in the spleen group; however, this difference was not statistically significant (P = 0.26) (Fig. 2D). The site of PTLD was GI tract (n = 1) and neck (n = 1) in the spleen group, and GI tract (n = 8), pharynx (n = 1) and kidney (n = 1) in the control group. The 2 PTLD cases in the spleen group (both adults) resulted in fatal consequences. Four of the 10 observed PTLD cases in the control group were among adults; each of these 10 patients responded to therapy. Observed incidence of PTLD in children was 0% (0 of 48) in the spleen group and 13.6% (6 of 44) in the control group (log-rank test, P = 0.06).
Hematological Complications
Autoimmune hemolysis (AIH) was observed in 5 patients in the spleen group (8.3%) and 1 patient in the control group (1.2%). Three of the 5 patients with AIH also developed idiopathic thrombocytopenic purpura (ITP) (Evans syndrome). Two of the AIH cases in the spleen group resulted in patient deaths. The single AIH case seen in the control group also had a fatal outcome. The observed mortality due to AIH was 3.3% (2 of 60) in the spleen and 1.2% (1 of 81) in the control groups. Two cases of autoimmune thrombocytopenia (ITP) without AIH were observed in the spleen group, while no case was seen in the control group. In one case, the ITP was refractory to therapy, and the patient died of an intracranial hemorrhage.
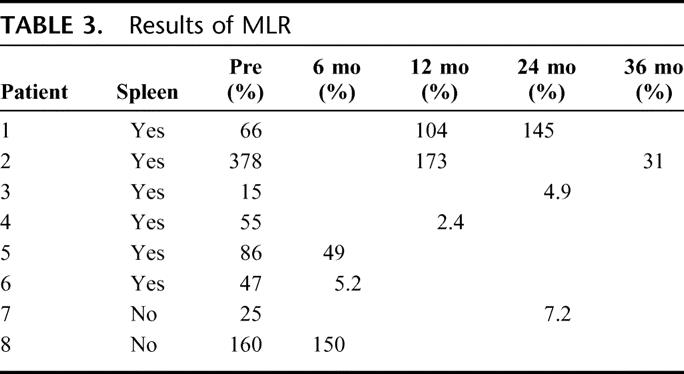
MLR
Among the patients who are currently alive, MLR assay results were available in 8 patients (6 in the spleen and 2 in the control groups, Table 3). Donor specific hyporesponsiveness (defined as the stimulation index against donor cells divided by the stimulation index against third party cells being <50%) was seen in 5 patients (83%) in the spleen group and one (50%) in the control group at 6 to 36 months postoperatively (Table 3).
TABLE 3. Results of MLR
Pneumococcal Titers
Antibody levels against S. pneumoniae were evaluated before and after vaccination in 3 patients in the spleen group. They all seemed to show an appropriate response (a 2- to 4-fold increase in type-specific antibody level 4 to 6 weeks postvaccination). Data for vaccination response was not available for patients in the control group.
Chimeric Levels
Peripheral blood lymphocyte chimerism was checked by flow-PCR methodology31 in 13 patients (9 in the spleen group, 4 in the control group). Multilineage lympohocyte chimerism was observed in all but 3 patients who showed no chimerism (detailed data not shown). Total chimeric levels (number of donor phenotype lymphocyte/total lymphocytes) ranged between 0% and 0.48% at 3 to 60 months follow up. No specific correlation with the allograft spleen was found in these preliminary results (total chimeric levels of these 13 patients are displayed in Table 6).
TABLE 6. Results of Peripheral Blood Lymphocyte Chimeric Level
Pathologic Findings of Transplanted Spleen
Three patients underwent allograft splenectomy for GVHD (n = 1), hemolytic anemia (n = 1), and ITP (n = 1) at 2, 8, and 1 month posttransplant, respectively. The transplanted spleen appeared grossly normal with no specific pathologic changes. No significant findings were noted in the transplanted spleens in deceased patients who underwent autopsy.
DISCUSSION
In animal models, transplantation of the spleen has been shown to induce donor-specific tolerance.7–13 This tolerogenic effect exists for itself as well as for other organs transplanted from the same donor. In 1974, Bitter-Suerman7 described spontaneous survival of the allograft spleen in certain major histocompatibility complex incompatible strain combinations in rats. Subsequent studies have shown the allogenic spleen can induce donor-specific tolerance to the skin, pancreas, and heart.8–10 Furthermore, there has been evidence that the potential mechanism of this effect derives from an induction of regulatory cells by the donor spleen.11–13 In a large animal model, Dor et al has recently shown that MLR and cell-mediated lympholysis (CML) responses to donor cells were suppressed with the inclusion of an allogenic splenic transplant in miniature swine.27
In the past, allogenic splenic transplantation was performed in humans for mainly 3 different settings (autotransplants as well but these are not included in this paper’s scope). The first setting was a combined pancreas and spleen transplant, which in the past has been the most commonly performed allogenic splenic transplant in humans. The spleen was used in this setting to ensure pancreatic blood flow, and no immunologic benefit from the allogenic spleen was reported.22–25 The second setting was the performance of a splenic transplant to treat hematological disorders such as hypogammaglubulinemia and hemophilia.14–19 Although recent reports have shown clinical improvement in the hematologic disease, most former attempts were not successful. The third setting was using a splenic transplant for terminal malignancies. It was performed for the potential effect of spleen to introduce an antitumor response (graft vs. tumor reaction), but these studies failed to show such an effect.20,21
MVT has now established its role in the treatment of abdominal catastrophes in both adults and children.5,6 In performing multivisceral transplants, the native spleen is removed by default; if the donor spleen is not transplanted, then the recipient is left in an asplenic state. Being in an asplenic state is a significant concern especially for a small child, since it has been associated with an increased risk of sepsis from encapsulated organisms in young children. We began including donor spleens in multivisceral transplants in 2001. We first started using the donor spleen in small children and then expanded this approach to adults. The purpose was to protect small children from the risk of sepsis associated with an asplenic state and to test its tolerogenic effect in both children and adults.
The grafted spleen clearly showed its function in normalizing recipient peripheral blood cell counts. In addition, although the patient numbers were small, donor spleen recipients showed a normal response to pneumococcal vaccination. Pathology of the grafted spleen was also available in a small number of cases. They all appeared grossly normal, and pathologic findings were not significant.
Acute rejection of the small bowel allograft continues to be a major cause of graft failure in small intestine transplantation. Comparing the rejection rates among isolated solid organ allografts, it would appear that the small bowel allograft has the highest rate of rejection as compared with other solid organs such as liver, kidney, heart, and pancreas. We previously reported that MVT has a protective effect regarding small bowel rejection.5,6 We suggested that part of the protective effect may be attributable to the effect of donor spleen.5
In this study, we analyzed the effect of donor spleen when it was transplanted as part of a multivisceral graft. The donor spleen seems to exhibit a protective effect on small bowel allograft rejection. In univariable analysis, inclusion of the donor spleen was among the factors favorably affecting freedom from any rejection and freedom from moderate or severe rejection. Inclusion of the donor spleen did not remain significant in multivariate analysis whereas factors such as transplant date ≥2001 (freedom from any rejection) and recipient age <2 (freedom from moderate or severe rejection) remained significant; however, there is a strong mutual association of transplant year ≥2001, recipient age <2 and receiving a spleen. Therefore, these results do not necessarily preclude an independent protective effect of spleen inclusion once the analysis is performed in a larger cohort and with longer follow up. In fact, the spleen group showed a trend toward superiority even among the patients whose date of transplant was ≥2001 (Figs. 1C–D, 2B–C). This trend was more apparent in children of age less than 1 (Figs. 1D, 2C). In particular, freedom from graft failure or death due to rejection appeared to be significantly improved in the spleen group among recipients age <1 whose date of transplant was ≥2001 (P = 0.03, Fig. 2C).
Preliminary results of MLR showed hypo- to unresponsiveness to donor cells in 5 tested patients who received a spleen and in 1 control group patient. Chimeric levels in the spleen and control groups did not appear different, and we do not have enough data from the control group to prove that this hyporesponsiveness is due to inclusion of the spleen: it could be a more general effect of the multivisceral graft rather than specifically from the spleen. There is increasing evidence that CD4+CD25+ T regulatory cells are involved in the mechanisms of immune tolerance.32,33 Composite intestinal grafts such as multivisceral grafts have more donor passenger leukocytes, as well as an increased nonhematopoietic cell mass. This introduction of such varied and large numbers of donor cells and their interaction with recipient immune cells may lead to an induction of immunosuppressive regulatory cell populations in patients with composite grafts, leading to a protective effect on the allograft from the recipient’s immune response. The allograft spleen may play an additional role in inducing regulatory cells. Another theory has suggested that clonal deletion was promoted by 2-way trafficking of recipient and donor cells,34 and the allograft spleen may play a role in such an interaction as well. Further study with more patients and longer follow up is underway at our institution to further clarify such mechanisms of tolerance in this patient population.
The analysis of infectious complications did not show any significant differences between the spleen and control groups. Only a slight trend towards a lesser average number of infectious episodes per patient in the spleen group was observed. However, this does not necessarily deny the transplanted spleen’s protective effect regarding sepsis from encapsulated organisms, since pneumococcal sepsis and meningococcemia were rarely observed overall (only 2 suspected cases in the entire cohort). In addition, most asplenic patients are kept on prophylactic penicillin for the rest of their lives, whereas we do not give penicillin prophylaxis to patients who receive splenic allografts. Although still preliminary, allogenic spleen recipients seem to show a normal response to pnuemococcal vaccination. Future studies of the serological response to the pneumococcal vaccination are necessary in clarifying this aspect of the effect of transplanting the donor spleen.
The original attempts to combine a spleen and pancreas transplant were abandoned due to reports of fatal GVHD. In this study, a slightly increased incidence of GVHD was observed in the spleen group (8.2% vs. 6.1% in the control group, respectively), but the difference was not statistically significant (P = 0.62). Thus, while GVHD remains as a significant concern in MVT, its occurrence does not appear to be specific to those who received a spleen.
Although there is no statistical difference, the observed incidence of PTLD seems to be less in the spleen group (3.3% vs. 12.3% in the control group, log-rank test P value = 0.32). On the other hand, the 2 cases of PTLD in the spleen group were fatal, whereas all 10 cases of PTLD in the control group responded to therapy. At this point we do not have a good explanation for this phenomenon. One possible explanation could be a graft versus tumor response of the spleen allograft as was proposed by previous researchers.20,21
Hematological complications after splenic transplantation may be a concern. We found 5 of 61 (8.2%) episodes of autoimmune hemolysis (2 of 5 were fatal) as compared with 1 of 81 (1.2%) in the control arm (this one case also had a fatal consequence). Autoimmune hemolysis has been reported in multivisceral transplants as well as in liver-intestine transplants and isolated intestinal transplants.5,35,36 In the latter 2 types of transplants, recipients retain their native spleen. The higher observed incidence in the spleen group as compared with the control group, which had no spleen, could be explained by the fact that splenectomy is a form of treatment for hemolysis. However, the potentially increased risk of hemolysis is a concern that will require further investigation and close monitoring.
In conclusion, MVT including donor spleen can be performed without significantly increasing the risk of GVHD. The allograft spleen showed its function in normalizing peripheral blood cell counts. The allogenic spleen seems to show some protective effect on small bowel rejection. We anticipate that further studies with longer follow up will help to clarify the immunologic and hematological effects of the allograft spleen.
Discussions
Dr. Goran B. Klintmalm (Dallas, Texas): It is rare to hear presentations on new procedures, especially procedures that provide benefits to our patients and also allows us to obtain new knowledge on biology.
When solid organ transplant in humans was first performed in the 1950s it suddenly gave us new views and hopes, and allowed us to gaze further due to the light spread by pioneering surgeons. Kidney transplantation, liver transplantation, pancreas transplantation, heart and lung transplantation, intestinal transplantation, and now we are being presented with a new concept—transplantation of the spleen for its biological effects.
Dr. Tzakis and his coworkers have just presented to us the first large study of spleen transplant recipients. The purpose behind the inclusion of the spleen allograft in the multivisceral graft was to alleviate the immunological deficit that the recipient suffers as a result of the splenectomy and to elucidate if a spleen allograft has a tolerogenic effect.
An intriguing finding is that the rejection seems to be less severe in the spleen recipients, which would support the tolerogenic hypothesis. However, the obvious danger that transplantation of the spleen could potentially bring is that of graft versus host disease and post-transplant lymphoproliferative disorder.
It is of note that there was no notable difference in the incidence of graft versus host disease in these patients. Whether this is surprising or not depends on which scientific theory you abide by regarding passenger leukocytes.
And this leads to my first question. You showed some engraftment studies on the recipients, but have you done it consistently in the two arms? What were the origin and the repertoire of the peripheral leukocytes in the stable successful recipient at one year, for example? To what extent were they chimeric? Did they have a fully functional repertoire?
Second, I find it interesting that even if statistically not significant, there was a noteworthy numerical difference in post-transplant lymphoproliferative disorders between the patients with a spleen and those without.
This is an intriguing finding, and thus my second question: Does a functioning spleen help protect us from the development of PTLD? What is your explanation for this intriguing finding?
Dr. Tomoaki Kato (Miami, Florida): Regarding your first question, we only measured lymphocyte chimerism in the peripheral blood. We have yet to study the recipient’s bone marrow, nor have we studied specific cell lineages separately.
Finally, we have not conducted a functional study of these cells. Our current protocol is to perform chimeric studies and immunological assays prospectively in recipients, including those who did and did not receive a spleen, and we hope to show these data in the near future.
As to your second question, fundamentally we still do not know exactly how inclusion of the spleen prevents PTLD. However, one of the theories, as proposed in the past by other researchers, is that the spleen may have the graft versus tumor effect, which could be from the various different cells that come with the splenic allograft.
Dr. Jean C. Emond (New York, New York): Operations of this type are obviously a virtuoso feat. They are also a biological feat probably unprecedented in medicine. These transplants are associated with a massive transfusion, if you will, of potentially immunologically capable cells and bone marrow precursors, and the idea of creating chimerism with this operation deliberately has been proposed before, leading to a spectrum between rejection, tolerance, and at the extreme, graft versus host disease. The notion of putting PTLD in that spectrum might also be relevant.
I think that the challenge for your team is to overcome the historical structure of this study. Between 1994 and today, almost 15 years, many things have changed. Surgery has improved. I know your group has been among the pioneers in advanced immunosuppression. You did not mention immunosuppression in your presentation. I think it would be worth discussing the possible effect on these results. The possibility is that donor recipient combinations, such as blood type, HLA and so forth, might also impact the value or detriment of the spleen in these operations.
Finally, I was wondering if you have any patients who received small bowel alone who have their own spleens, I know it is another difficult comparison, but whether there is a difference between the allograft spleen and the host spleen in terms of immune function.
Dr. Tomoaki Kato (Miami, Florida): Regarding your first question about immunosuppression protocols, yes, you are right that I have not shown the immunosuppressive protocol. For children, since 1998 till now, the protocol has changed little. In adults, however, since 2001 Campath 1H was introduced in the immunosuppressive protocol. We looked at the effect of Campath 1H and also at the other induction immunosuppressive protocols in univariate and multivariate analyses. There are no differences between each induction therapy. The only difference we found was that patients who received no induction therapy had a higher rate of rejection.
As to your second question, we have not found that HLA matching or blood type impacts on the hazard rate of developing rejection.
Finally, regarding your last question, we have not systematically studied the function of the native spleen in patients who received isolated intestinal transplant. Nonetheless, it certainly would be interesting to compare the function of the allograft spleen to that of a normal, native spleen.
Dr. Raymond Pollak (Naperville, Illinois): The premise or experimental basis for this paper is 20 years old, when we transplanted the spleen with the heart in rodent models. And in those models we were able to show using adoptive transfer experiments that tolerance was a phenomenon mediated by cells with a suppressor cell phenotype. So my first question is: have you looked for suppressor cell phenotypes in the recipients, because they are not thought to exist in man? And thinking about that, how would you explain this phenomenon biologically?
Secondly, van Rood and others showed many years ago that graft tolerance mediated by the infusion of hemopoeitic cells was either a population selection phenomenon, or could be induced only when donor and recipient shared an HLA allele. So have you HLA-typed your donors and recipients and is there any histocompatibility data to explain this phenomenon?
Dr. Tomoaki Kato (Miami, Florida): Regarding your first question, I guess the suppressive phenotype of cells you refer to is the T regulatory cells. Preliminary, we have looked at some of the regulatory population in recipients who received a spleen, but I am afraid that the data is not ready for presentation. We are working on a hypothesis that the effect of the spleen might be due to the regulatory cell.
As to your second question, regarding splenocyte infusion and HLA typing, our data suggest that HLA matching would not impact the results.
Dr. Mark D. Pescovitz (Indianapolis, Indiana): I have several questions, one that may reach beyond the scope, but it will get into a little bit of the ethics or the philosophy of this approach.
First, you immunized the patients with pneumococcal antigen as a test of splenic function. But in point of fact, the immunization is an intramuscular injection and is therefore obviating any splenic function since you are not having the antigen pass through the spleen, as the pneumococcal bacteria might. Have you contemplated any other sort of immunization strategies, such as bacteriphage phi-174, which is given intravenously, as an assessment of splenic function?
The second question, you report that there was no graft versus host disease but you report three cases of what you call autoimmune disease. So when does the organ become part of the patient so that it is auto as opposed to allo? Therefore, is your “autoimmune” disease really an alloimmune graft versus host disease? There have clearly been cases of alloimmune thrombocytopenia and anemia after transplant.
And my last question sort of gets at the ethics of splenic transplant. People have been talking about splenic transplantation for a while. Your data suggest that there may be an effect there. Other people have talked about using it in the setting of kidney transplants as well. The cadaver donor only comes with one spleen. If your idea works out, how are you going to regulate who gets what organs? While that may be beyond your scope, it is an interesting question I have had.
Dr. Tomoaki Kato (Miami, Florida): Regarding your first question, we have not looked at response to other forms of vaccination.
With regard to GVHD, there was no difference in incidence between the splenic and control groups. However, the incidence of autoimmune disease was significantly higher in the spleen group. This might be a form of graft versus host disease. This is a very important question. We still have not found an answer. The development of autoimmunity has been reported in other types of transplants. This might have something to do with an immunosuppressive effect that suppresses regulatory cells leading to the development of autoimmune disease.
Footnotes
Reprints: Tomoaki Kato, MD, Department of Surgery, Division of Transplantation, University of Miami, 1801 NW 9th Avenue, 5th Floor, Miami, FL 33136. E-mail: tkato@med.miami.edu.
REFERENCES
- 1.Hansen K, Singer DB. Asplenic-hyposplenic overwhelming sepsis: postsplenectomy sepsis revisited. Pediatr Dev Pathol. 2001;4:104–121. [DOI] [PubMed] [Google Scholar]
- 2.Spencer J, Perry ME, Dunn-Walters DK. Human marginal-zone B cells. Immunol Today. 1998;19:421–426. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan JL, Ochs HD, Schiffman G, et al. Immune response after splenectomy. Lancet. 1978;1:178–181. [DOI] [PubMed] [Google Scholar]
- 4.Leonard AS, Giebink GS, Baesl TJ, et al. The overwhelming postsplenectomy sepsis problem. World J Surg. 1980;4:423–432. [DOI] [PubMed] [Google Scholar]
- 5.Tzakis AG, Kato T, Levi DM, et al. 100 multivisceral transplants at a single center. Ann Surg. 2005;242:480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato T, Tzakis A, Selvaggi G, et al. Intestinal and mutivisceral transplantation in children. Ann Surg. 2006;243:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitter-Suermann H. Survival of unmodified spleen allografts in rats. Nature. 1974;247:465–466. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Li XH, Miyamoto M, et al. Induction of transplantation tolerance in adult rats by vascularized spleen transplantation. Transplantation. 1997;64:650–654. [DOI] [PubMed] [Google Scholar]
- 9.Bitter-Suermann H. Prolonged survival of auxiliary spleen allografts in rats inducing acceptance of skin allografts. Transplantation. 1974;17:75–83. [DOI] [PubMed] [Google Scholar]
- 10.Bitter-Suermann H, Save-Soderbergh J. The course of pancreas allografts in rats conditioned by spleen allografts. Transplantation. 1978;26:28–34. [DOI] [PubMed] [Google Scholar]
- 11.Duncan WR, Stepkowski SM, Bitter-Suermann H. Induction of transplantation in rats by spleen allografts. I. Evidence that rats tolerant of spleen allografts contain two fenotypically distinct T suppressor cells. Transplantation. 1986;41:626–633. [DOI] [PubMed] [Google Scholar]
- 12.Duncan WR, Bitter-Suermann H, Stepkowski SM. Induction of transplantation tolerance in rats by spleen allografts. III. The role of T suppressor cells in the induction of specific unresponsiveness. Transplantation. 1987;44:553. [DOI] [PubMed] [Google Scholar]
- 13.Stepkowski SM. Bitter-Suermann H. Dukan WR. Evidence supporting an in vivo role for T suppressor cells in spleen allograft induced tolerance. Transplant Proc. 1986;18:207. [Google Scholar]
- 14.Marchioro TL, Rowlands DT, Rifkind D, et al. Splenic homotransplantation. Ann NY Acad Sci. 1964;120:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hathaway WE, Mull MM, Githens JH, et al. Attempted spleen transplant in classical hemophilia. Transplantation. 1969;7:73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia SS, Jiang HC, Zhou XX, He G. Treatment of hemophilia A by living mother-to-son splenic transplantation. First case report in the world. Chin Med J (Engl). 1992;105:609–611. [PubMed] [Google Scholar]
- 17.Xiang WZ, Jie ZW, Sheng XS. Clinical observation on hemophilia A treatment by cadaveric spleen transplantation. Transplant Proc. 2002;34:1929–1931. [DOI] [PubMed] [Google Scholar]
- 18.Groth CG, Blomstrand R, Dreborg S, et al. Splenic transplantation in Gaucher disease. Birth Defects Orig Artic Ser. 1973;9:102–105. [PubMed] [Google Scholar]
- 19.Groth CG, Dreborg S, Ockerman PA, et al. Splenic transplantation in a case of Gaucher’s disease. Lancet. 1971;1:1260–1264. [DOI] [PubMed] [Google Scholar]
- 20.Raccuglia G, Lansing A. Spleen transplantation in a leukaemic individual from his healthy identical twin. Clin Exp Immunol. 1973;14:1–18. [PMC free article] [PubMed] [Google Scholar]
- 21.Liu DL, Xia S, Tang J, et al. Allotransplantation of whole spleen in patients with hepatic malignant tumors or hemophilia A. Operative technique and preliminary results. Arch Surg. 1995;130:33–39. [DOI] [PubMed] [Google Scholar]
- 22.Starzl TE, Iwatsuki S, Shaw BW Jr, et al. Pancreaticoduodenal transplantation in humans. Surg Gynecol Obstet. 1984;159:26S. [PMC free article] [PubMed] [Google Scholar]
- 23.Deierhoi MH, Sollinger HW, Bozdech MJ, et al. Lethal graft-versus-host disease in a recipient of a pancreas spleen transplant. Transplantation. 1986;41:544–546. [PubMed] [Google Scholar]
- 24.Gonwa TA, Goeken NE, Schulak JA, et al. Failure of cyclosporine to prevent in vivo T cell priming in man. Studies in allogeneic spleen transplantation. Transplantation. 1985;40:299–304. [DOI] [PubMed] [Google Scholar]
- 25.Booster MH, Wijnen RM, van Hooff JP, et al. The role of the spleen in pancreas transplantation. Transplantation. 1993;56:1098–1102. [DOI] [PubMed] [Google Scholar]
- 26.Dor FJMF, Gollackner B, Cooper DKC. Can spleen transplantation induce tolerance? A review of the literature. Transpl Int. 2003;16:451–460. [DOI] [PubMed] [Google Scholar]
- 27.Dor FJMF, Cooper DKC. Immunologic benefits of spleen transplantation in the absence of graft-versus-host disease. Ann Surg. 2006;243:710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Ruiz P, Thompson J, et al. Intestinal and multivisceral transplantation. Word J Surg. 2002;26:226–237. [DOI] [PubMed] [Google Scholar]
- 29.Kato T, Tzakis A. Transplantation of the liver with digestive organs. In: Busuttil, Klintmalm, eds. Transplantation of Liver. 2nd ed. Philadelphia, PA: Elsevier-Saunders; 2005:787–802. [Google Scholar]
- 30.Mittal NK, Tzakis AG, Kato T, et al. Current status of small bowel transplantation in children: update 2003. Pediatr Clin North Am. 2003;50:1419–1433. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Morales R, Carreno M, Mathew JM, et al. The effects of chimeric cells following donor bone marrow infusions as detected by PCR-flow assays in kidney transplant recipients. J Clin Invest. 1997;99:1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fehervari Z, Sakaguchi S. CD4+ T regs and immune control. J Clin Invest. 2004;114:1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. [DOI] [PubMed] [Google Scholar]
- 34.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panaro F, DeChristopher PJ, Rondelli D, et al. Severe hemolytic anemia due to passenger lymphocytes after living-related bowel transplant. Clin Transplant. 2004;18:332–335. [DOI] [PubMed] [Google Scholar]
- 36.Brunner B, Kropshofer G, Ellemunter H, et al. Severe cold agglutinin disease caused by recurrent monomorphic Epstein-Barr virus (EBV)-associated post-transplant lymphoproliferative disorder (PTLD), clonally related to an EBV-negative plasmacytic hyperplasia in a pediatric multivisceral organ transplant recipient. Pediatr transplant. 2007; doi:10. 1111/j. 1399–3046. 2007. 00711. [DOI] [PubMed]