Abstract
Background:
The extent of surgery for papillary thyroid cancers (PTC) remains controversial. Consensus guidelines have recommended total thyroidectomy for PTC ≥1 cm; however, no study has supported this recommendation based on a survival advantage. The objective of this study was to examine whether the extent of surgery affects outcomes for PTC and to determine whether a size threshold could be identified above which total thyroidectomy is associated with improved outcomes.
Methods:
From the National Cancer Data Base (1985–1998), 52,173 patients underwent surgery for PTC. Survival was estimated by the Kaplan-Meier method and compared using log-rank tests. Cox Proportional Hazards modeling stratified by tumor size was used to assess the impact of surgical extent on outcomes and to identify a tumor size threshold above which total thyroidectomy is associated with an improvement in recurrence and long-term survival rates.
Results:
Of the 52,173 patients, 43,227 (82.9%) underwent total thyroidectomy, and 8946 (17.1%) underwent lobectomy. For PTC <1 cm extent of surgery did not impact recurrence or survival (P = 0.24, P = 0.83). For tumors ≥1 cm, lobectomy resulted in higher risk of recurrence and death (P = 0.04, P = 0.009). To minimize the influence of larger tumors, 1 to 2 cm lesions were examined separately: lobectomy again resulted in a higher risk of recurrence and death (P = 0.04, P = 0.04).
Conclusions:
The results of this study demonstrate that total thyroidectomy results in lower recurrence rates and improved survival for PTC ≥1.0 cm compared with lobectomy. This is the first study to demonstrate that total thyroidectomy for PTC ≥1.0 cm improves outcomes.
Extent of surgery for papillary thyroid cancers (PTC) remains controversial. The results of this study demonstrate that total thyroidectomy is associated with lower recurrence rates and improved survival for PTC ≥1.0 cm compared to lobectomy. This is the first study to demonstrate that total thyroidectomy for PTC ≥1.0 cm improves outcomes.
Papillary thyroid cancer (PTC) is the most common but least aggressive histologic subtype of thyroid cancer. The American Cancer Society estimates that approximately 33,550 people in the United States will be diagnosed with thyroid cancer in 2007, and 1530 will die of the disease.1 Most patients with PTC have an excellent prognosis. However, recent studies have demonstrated an increasing incidence of PTC, particularly due to the detection of tumors <2 cm.2,3 Moreover, mortality rates may be increasing for certain subgroups.4
For decades, there has been considerable debate regarding the extent of surgery for PTC, particularly for tumors <4 cm in diameter.5–7 Recent consensus guidelines suggest that patients with tumors displaying high-risk features should undergo a total or near-total thyroidectomy, and patients with single, small, low-risk, intrathyroidal node-negative PTC may be candidates for less extensive surgery such as a lobectomy with or without isthmusectomy.8,9 These guidelines, based on retrospective studies and general consensus from experts, recommend near-total or total thyroidectomy for PTC > 1.0 to 1.5 cm. However, a difference in survival favoring total thyroidectomy over lobectomy has only been demonstrated for tumors >3 cm.10–12 Thus, these guidelines advocating near-total or total thyroidectomy for PTC ≥1.0 cm are not based on data demonstrating a difference in survival, but rather on the possibility of multifocal disease, recurrence, and other practicalities of treatment and follow up.6,13 Prospective randomized clinical trials assessing the impact of surgical management on PTC outcomes are impractical and have not been performed because they would require a large number of patients to be followed for an extended period of time.14
We hypothesized that total thyroidectomy for PTC results in improved outcomes compared with lobectomy. Using the National Cancer Data Base (NCDB), we identified 52,173 patients who underwent surgery for PTC in the United States from 1985 to 1998. The objective of this study was to determine whether total thyroidectomy resulted in improved recurrence and long-term survival rates for patients with PTC. Furthermore, we sought to determine whether a specific tumor size threshold could be identified above which total thyroidectomy was associated with a decreased risk of recurrence and death.
PATIENTS AND METHODS
Data Acquisition and Study Population
The NCDB is a program of the American College of Surgeons and the Commission on Cancer.15 The NCDB has been accruing data since 1985 and captures newly diagnosed malignancies from over 1440 Commission on Cancer approved hospitals. These hospitals account for more than 75% of all new cancers in the United States each year. The NCDB collects data regarding patient demographics, tumor characteristics, preoperative and postoperative staging, treatment details, recurrence, and survival. Health systems data, provider information, and socioeconomic status are obtained through linkage to tertiary data sources. Based on national incidence estimates from the American Cancer Society, the NCDB captures approximately 88% of all new thyroid cancers annually in the United States.16
Using the NCDB, patients from 1985 to 1998 were identified using the International Classification of Disease—Oncology, 2nd Edition (ICD-O-2) code for thyroid malignancies: C73.9.17 Diagnosis year 1998 was the most recent year available with at least 5 years of follow-up data. Patients were excluded if they had histologies other than PTC (ICD-O-2 codes 8050 and 8260), had poorly differentiated tumors (reported grade 3 or 4), were younger than 18 years old, had missing tumor size measurements, or did not undergo surgery. Patients who underwent surgery for PTC were identified based on the Registry Operations and Data Standards site-specific procedure coding.18 For this study, patients were dichotomized by extent of surgery into 1 of 2 heterogeneous groups to distinguish bilateral versus unilateral resection: “total thyroidectomy” (including total, near-total, or subtotal) or “lobectomy” (including lobectomy with or without isthmusectomy). Patients who underwent removal of less than a lobe (“nodulectomy”) (n = 6381) or who were classified as “thyroidectomy, not otherwise specified (NOS)” (n = 7632) were excluded. If a patient initially underwent lobectomy and then went on to have a completion thyroidectomy, the patient was recorded in the NCDB as having undergone a total thyroidectomy.19
Statistical Analysis
Descriptive statistics were calculated for all variables. Ten-year recurrence and survival rates were calculated as the time from surgery to recurrence or death, respectively. Recurrence of disease was defined as any locoregional or distant recurrence after a documented disease-free period. Patients who had metastases at presentation or were never disease free after surgery were excluded from the recurrence analysis. Outcomes were estimated by the Kaplan-Meier method and compared using the log-rank test.20 Relative overall survival was calculated by adjusting the observed survival rates for differences in gender, age, and race using 2000 US Census Bureau data.21 Median follow-up was 69.7 months. At 5 years from surgery, 27,776 patients were uncensored (alive with follow-up data) in the total thyroidectomy group and 6429 patients were uncensored in the lobectomy group. At 10 years from surgery, 8531 patients were uncensored for total thyroidectomy and 2493 patients were uncensored for lobectomy. Cox Proportional Hazards modeling was used to evaluate the association of tumor size and extent of surgery on recurrence and survival while adjusting for potential confounders including gender, age (<45, 45–64, ≥65 years), race (White, Black, Asian, Hispanic, Other), annual income (<$36,000 vs. ≥$36,000), absence or presence of nodal metastases, absence or presence of distant metastases, radioactive iodine (RAI) administration, year of diagnosis (1985–1993 vs. 1994–1998), and hospital volume.22
Tumor size cutoffs from 4.0 cm to 0.5 cm in 0.5 cm intervals were examined to determine whether a tumor size threshold could be determined above which total thyroidectomy results in improved survival. Stratified analyses using the Cox Proportional Hazards model were performed for tumors <1.0 cm, ≥1.0 cm, 1.0 to 1.9 cm, and 2.0 to 4.0 cm in greatest diameter. The analysis was further stratified by age <45 years and ≥45 years, but as the results were qualitatively similar, the results are not shown. Before analysis, all independent variables in the model were examined by calculating a Pearson correlation coefficient. We tested for interactions between tumor size, extent of surgery, and patient age. The Proportional Hazards assumptions were confirmed graphically. Hazard Ratios with 95% Confidence Intervals were generated. Hazard Ratios >1.0 indicate an increased risk of death.
Procedure volume quartiles were calculated based on average annual thyroid surgery volume with equal numbers of hospitals distributed within the quartiles. Since patient-level socioeconomic data is not collected by the NCDB, median household income was assessed at the zip-code level based on the patient’s residence at the time of diagnosis using 2000 United States Census Bureau data.23 The level of statistical significance was set to P < 0.05. All P values reported are 2-tailed. Statistical analyses were performed using SPSS, version 14 (Chicago, IL). This study was reviewed by the Northwestern University Institutional Review Board.
RESULTS
Of 66,957 patients with PTC in the NCDB from 1985 to 1998, 52,173 underwent operations that could be classified as either total thyroidectomy (bilateral resection including total, near-total, or subtotal) or lobectomy (unilateral resection including lobectomy with or without isthmusectomy), and had complete information on tumor size (Table 1). Of the 52,173 patients, 43,227 (82.9%) underwent total thyroidectomy, and 8946 (17.1%) underwent lobectomy. Of patients who underwent surgery for PTC, 23.9% had tumors <1 cm; 29.8% had tumors 1 to 2 cm; and 46.3% had tumors >2 cm. Nodal metastases were present in 34.6% of patients who had nodes examined, and distant metastases were present in 2.2% of patients at diagnosis (excluding the MX [unknown metastases] patients). RAI administration was reported for 56.2% of patients who underwent total thyroidectomy.
TABLE 1. Characteristics of Patients Who Underwent Surgery for PTC
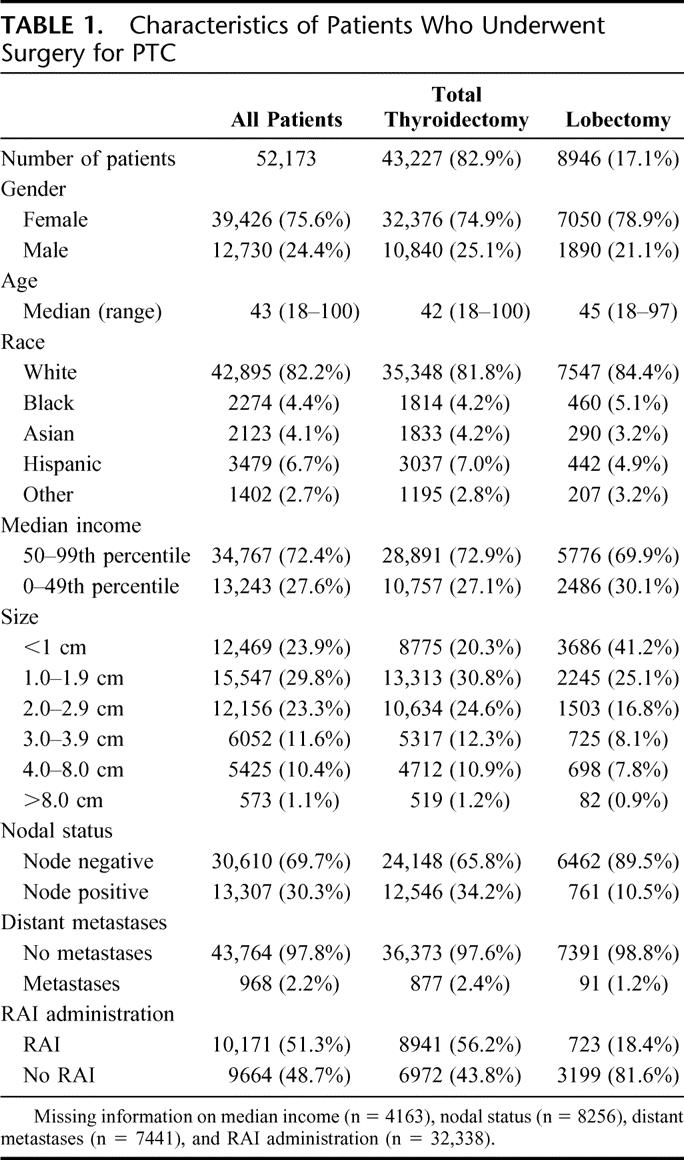
For all patients with PTC, the recurrence rates were 5.7% at 5 years and 9.4% at 10 years. Recurrence rates were compared by tumor size and extent of surgery (Fig. 1). Ten-year recurrence rates increased with increasing tumor size: <1.0 cm 4.6%, 1.0 to 1.9 cm 7.1%, 2.0 to 2.9 cm 8.6%, 3.0 to 3.9 cm 11.6%, 4.0 to 8.0 cm 17.2%, and >8.0 cm 24.8% (P < 0.0001 for each pairwise comparison). When examining all tumor sizes together using univariate methods, patients who underwent total thyroidectomy had an unadjusted 10-year recurrence rate of 7.7%; whereas, patients who underwent lobectomy had an unadjusted 10-year recurrence rate of 9.8% (P < 0.05).
FIGURE 1. Recurrence rates after surgery for patients with PTC (A) by tumor size and (B) by extent of surgery.
Survival rates were compared by tumor size and extent of surgery (Fig. 2). Ten-year survival rates declined with increasing tumor size, but survival was statistically worse only for tumors larger than 4.0 cm (P < 0.0001): <1.0 cm 98.0%, 1.0 to 1.9 cm 98.4%, 2.0 to 2.9 cm 98.5%, 3.0 to 3.9 cm 95.5%, 4.0 to 8.0 cm 90.5%, and >8.0 cm 81.3%. When examining all tumor sizes together using univariate methods, 10-year survival was higher for patients who underwent total thyroidectomy compared with lobectomy: 98.4% versus 97.1% (P < 0.05).
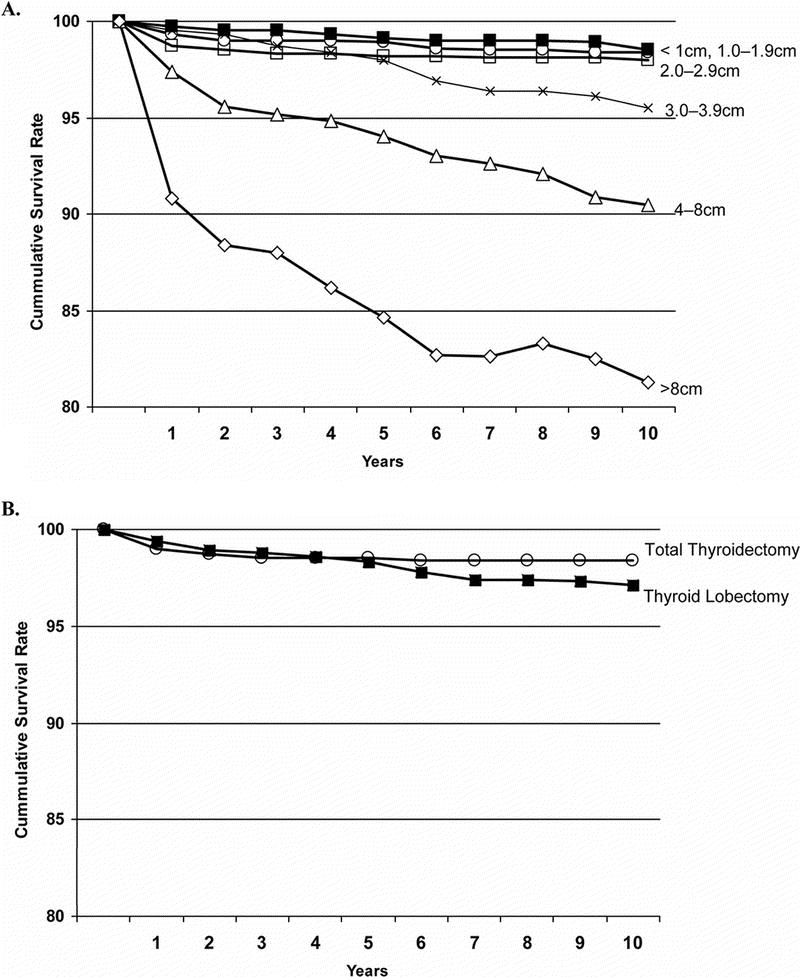
FIGURE 2. Relative survival rates after surgery for patients with PTC (A) by tumor size and (B) by extent of surgery.
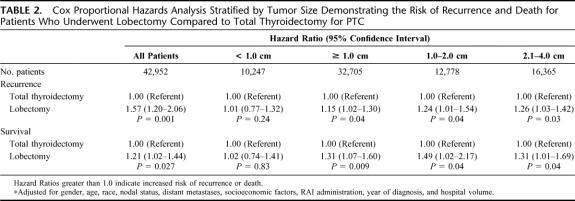
Multivariate analyses using Cox Proportional Hazards modeling were used to investigate the impact of tumor size and extent of surgery on recurrence and survival while controlling for patient, tumor, treatment, and hospital characteristics. In the evaluation of all patients, lobectomy was associated with a 57% higher risk of recurrence and a 21% higher risk of death compared with total thyroidectomy (P = 0.001 and, P = 0.027, respectively) (Table 2). To determine the tumor size threshold where extent of surgery affects outcomes, the Cox Proportional Hazards analysis was stratified by tumor size: <1.0 cm, ≥1.0 cm, 1.0 to 1.9 cm, or 2.0 to 4.0 cm. For patients with tumors <1.0 cm, there was no difference in recurrence or survival between total thyroidectomy and lobectomy. However, for patients with tumors ≥1.0 cm, lobectomy was associated with a 15% higher risk of recurrence and 31% higher risk of death (P = 0.04 and P = 0.04, respectively). To address the potential confounding effect of larger tumors when examining all tumors ≥1.0 cm, 1.0 to 2.0 cm tumors were examined separately. Compared with patients who underwent total thyroidectomy for a 1.0 to 2.0 cm malignancy, those undergoing lobectomy had a 24% higher risk of recurrence and a 49% higher risk of death (P = 0.04 and P = 0.04, respectively).
TABLE 2. Cox Proportional Hazards Analysis Stratified by Tumor Size Demonstrating the Risk of Recurrence and Death for Patients Who Underwent Lobectomy Compared to Total Thyroidectomy for PTC
DISCUSSION
Consensus guidelines recommend near-total or total thyroidectomy for PTC 1.0 to 1.5 cm in diameter or larger; however, these recommendations have not been based on a demonstrated survival difference.5,9 The objective of this study was to examine whether the extent of surgery affects outcomes for PTC and to determine whether a size threshold could be identified above which total thyroidectomy is associated with improved outcomes. Patients were dichotomized into 2 broad categories: “total thyroidectomy” (bilateral resection including total, near-total, and subtotal) and “lobectomy” (unilateral resection including lobectomy with or without isthmusectomy). Using multivariate modeling controlling for confounding factors, we evaluated the impact of total thyroidectomy compared with lobectomy on recurrence and survival for various tumor sizes.
Our first objective was to determine whether the extent of surgery was associated with outcomes for all patients with PTC irrespective of tumor size. Considerable controversy surrounds the extent of surgery for PTC.5,6 Recommendations regarding the extent of surgery are largely based on nonrandomized, retrospective studies from single institutions.9,10,12,13,20,24–29 Although these single institutional studies are able to perform pathologic reviews of specimens and likely have more accurate and detailed data than cancer registries, the ability to study this question with multivariate methods has been limited by the large sample size required to demonstrate differences in outcomes for PTC. A power analysis performed by Udelsman et al found that between 3100 and 10,400 patients with mean follow up longer than 6 years would be needed to study survival differences between total thyroidectomy and lobectomy.14 The largest reported patient population addressing this question before our study was 4402 patients from the National Cancer Institute’s Surveillance Epidemiology and End Results database.30 In that study, Haigh et al stratified patients into low and high-risk groups based on the Age, Metastases, Extent, and Size prognostic criteria and found no improvement in survival after total thyroidectomy for high and low-risk patients. A recent study of 2936 cases from the National Thyroid Cancer Treatment Cooperative Study Group found that patients undergoing total thyroidectomy for Stage III or IV differentiated thyroid cancer had improved overall survival; however, they found no difference in disease-free survival based on extent of surgery regardless of Stage grouping.31 In our examination of all 52,173 patients, we found that total thyroidectomy was associated with a lower risk of recurrence and death compared with lobectomy.
Our second objective was to determine whether a tumor size threshold could be identified above which total thyroidectomy was associated with improved recurrence and survival. Consensus guidelines suggest that tumors displaying high-risk features merit more aggressive surgery consisting of a total or near-total thyroidectomy, and patients with isolated, small, low-risk, intrathyroidal, node-negative tumors may be candidates for less extensive surgery such as a lobectomy.8,9,32 The challenge rests in classifying these tumors into high and low-risk groups. No less than 15 classification schemes have been proposed attempting to identify specific high-risk features such as gender, age, size, metastases, extrathyroidal extension, and grade.33 However, these studies have been based on institutional experiences consisting of relatively small patient cohorts at tertiary referral centers and vary in their identified prognostic factors. In particular, it has been difficult to identify a tumor size threshold above which patients should undergo a total thyroidectomy. In 1990, DeGroot et al20 reported their experience with 269 PTC patients and demonstrated a benefit in recurrence and long-term survival for total thyroidectomy over lobectomy in patients with tumors 1.0 cm or larger; however, 64% of their lobectomy comparison group had patients who may have undergone a procedure less than a lobectomy (ie, nodulectomy), which would likely bias the outcomes in favor of total thyroidectomy. To analyze tumor size thresholds, a sufficient number of patients must be available in each subgroup. With the large sample size available through national cancer registries, subgroup analyses are feasible, as in this study where there were 12,778 patients in the 1.0 to 2.0 cm group. We assessed various size cutoffs, starting from 4.0 cm decreasing in 0.5 cm intervals to 0.5 cm in diameter, to determine whether a tumor size threshold could be determined above which total thyroidectomy was associated with a lower risk of death. On univariate analysis, the threshold appeared to be 4 cm in greatest diameter based on the estimates by the Kaplan-Meier method and log-rank test, but using a multivariate Cox Proportional Hazards model, we demonstrated that total thyroidectomy is associated with a lower risk of recurrence and death compared with lobectomy for patients with tumors ≥1.0 cm.
Various systems classifying tumors into high and low-risk groups include tumor size as a one of the prognostic grouping factors, but size thresholds range widely from 1.0 to 5.0 cm.10,27,34–36 Guidelines based on retrospective studies and general consensus from experts recommend total thyroidectomy for tumors larger than 1.0 to 1.5 cm.8,9 These recommendations advocating total thyroidectomy are based on several factors including (1) the high frequency of multifocal disease, (2) the facilitation of RAI ablation of remaining thyroid tissue, (3) the opportunity to follow patients with thyroglobulin levels and 131I scans, and (4) the avoidance of reoperative neck surgery.5,7,13 Opponents argue that total thyroidectomy for these smaller tumors will not affect survival and will lead to an increased frequency of surgical complications such as recurrent laryngeal nerve injury and permanent hypocalcemia.5,6 A difference in overall survival favoring total thyroidectomy has only been demonstrated for tumors ≥3 cm.10,12 Thus the established guidelines advocating total thyroidectomy for PTC larger than 1.0 to 1.5 cm are not based on data demonstrating a difference in survival, but rather on other practical considerations of treatment and follow up. The results of our study demonstrate that total thyroidectomy for PTC is associated with a lower risk of recurrence and death for cancers ≥1.0 cm. For PTC <1.0 cm, there is no difference in outcomes between total thyroidectomy and lobectomy.
This study should be interpreted in light of several potential limitations. First, analyses using administrative databases and cancer registries are limited by the information collected, and missing variables may lead to biased results. For example, the NCDB does not collect data on postoperative thyroid-stimulating hormone suppression therapy, thus this factor could not be included in our outcomes analyses. Furthermore, some studies have demonstrated that extrathyroidal extension is an important prognostic factor, but this information was also not available in the NCDB at the time of the study; however, this variable is being collected by the NCDB as of 2004. Furthermore, it is not possible or feasible to perform a pathologic review for all patients in this study or verify the NCDB data using patient charts. Thus, single-institution studies are valuable and offer an alternate perspective to analyses using cancer registry data.
An additional limitation is that only 56.2% of patients who underwent a total thyroidectomy were reported to have received RAI in our study. A recent study from the National Thyroid Cancer Treatment Cooperative Study Group (1987–2001) reported a RAI utilization rate of 70%.31 However, these data come from hospital chart abstractions from institutions that focus on thyroid cancer management, potentially explaining the higher observed utilization rate. Studies attempting to quantify the reporting disparity by comparing registry data to patient charts and Medicare claims have shown that the underreporting ranges from 5% to 12% for adjuvant radiation and chemotherapy.37–40 Furthermore, the observation that lobectomy patients are reported to receive RAI may stem from several explanations. First, we know anecdotally that there are physicians administering RAI improperly. Secondly, it may be that the surgery is coded incorrectly; however, this is highly unlikely as numerous studies have demonstrated that procedure coding in cancer registries and administrative datasets is highly accurate.41 Finally, it is conceivable that cancer registrars are recording diagnostic 131I scans inaccurately as therapeutic RAI. The NCDB can be used to identify reasons why patients who undergo a lobectomy receive RAI. When a hospital reports such a case, the record will be returned to the hospital, and they will be required to verify the data and provide an explanation for why a lobectomy patient received RAI. Thus, we will be able to determine whether it is a matter of improper RAI administration, incorrect procedure coding, or inaccurate coding of therapeutic RAI versus 131I scans.
Another potential limitation is that the hospitals that are included in the NCDB must be an approved Commission on Cancer hospitals.42 Thus a potential selection bias may exist as centers included in the NCDB may have a higher level of oncologic specialization than hospitals in the United States that do not report to the NCDB. These hospitals may have different treatment strategies, complication rates, and outcomes for PTC. In addition, determination of disease-specific survival is limited in cancer registry data. The NCDB captures the cause of death from hospital registries. Thus, the actual cause of death due to cancer is notably underreported.43 As a consequence, we used relative survival which is the observed survival rate divided by the expected survival for a cohort similar in age, ethnicity, and sex (presumably in the absence of thyroid cancer) using 2000 US Census Bureau data.21 Relative survival currently serves as the best estimate of disease-specific survival when using cancer registry data. One may speculate that the worse survival observed for thyroid lobectomy is a result of patients with advanced age and severe comorbidities being selected to undergo lobectomy; however, we adjusted for patient, tumor, treatment, and hospitals factors in our multivariate models in an attempt to control for patient selection issues.
Finally, without data on national complication rates after thyroid surgery, one should be concerned about recommending total thyroidectomy for all patients with PTC ≥1.0 cm.44 Surgeons performing thyroid operations infrequently at low-volume centers may choose to offer a unilateral operation (ie, lobectomy) rather than a bilateral operation such as near-total or total thyroidectomy to avoid the possibility of bilateral nerve injury or permanent hypocalcemia. In experienced hands, the frequency of permanent recurrent laryngeal nerve injury or permanent hypocalcemia should be less than 2.0%, and in 1 study has been shown to be 0.4% and 0.2% respectively for high-volume surgeons.44 The modest outcome benefit demonstrated in this study must be weighed cautiously against the potential complications after total thyroidectomy and interpreted in the context of an individual surgeon’s experience and complication rates. If a surgeon’s complication rate is more than 1% to 2%, thyroid lobectomy may serve the patient with low-risk PTC better and supersede the improvement in outcomes with total thyroidectomy. Alternatively, referral to a thyroid surgeon with low complication rates should be considered whenever feasible.
CONCLUSION
This is the largest study on PTC to date, and the first to clearly demonstrate a survival benefit for total thyroidectomy in tumors ≥1.0 cm, even after adjusting for other low-risk features. No prior study has specifically attempted to examine the extent of surgery for thyroid cancer to determine a size threshold which is associated with improved outcomes. Thus, we have provided data supporting total thyroidectomy for PTC ≥1.0 cm on the basis of improvements in recurrence and long-term survival.
ACKNOWLEDGMENTS
The authors thank the staff of the American College of Surgeons, National Cancer Data Base for their assistance, particularly James Banasiak, MS and E. Greer Gay, RN, MPH, PhD.
Discussions
Dr. Gerard M. Doherty (Ann Arbor, Michigan): I think this is a very important study in clinical thyroidology and will probably answer some questions once and for all. I think the real strength of the study is that you have taken the “large simple study” approach to the randomized clinical trial that we are not able to do and demonstrated a very clear outcome difference in survival that I believe will stand the test of time.
I have 2 questions and 1 comment. The questions anticipate the criticisms that will come with this study. This work will be very carefully dissected in the American Thyroid Association and in other venues.
The first question is how do you think the data errors and gaps that exist in the National Cancer Database have affected the outcome, and how do you think they have skewed the results? It is clear that when you use a clinical database that includes input from over 20 years and over 1400 hospitals, there will be mistakes embedded in the data. And, there are some obvious errors from the data that you report. For instance, patients who have had a thyroid lobectomy alone are not treated with radioactive iodine ablation, and yet almost 20% of the patients in your thyroid lobectomy group had radioiodine reported as part of the therapy. They likely received a thyroid lobectomy elsewhere in a non-NCDB institution, so you only have records of the completion thyroidectomy and they have thus contaminated the lobectomy group. How do you think that affects the study findings?
Similarly, with the information regarding lymph nodes, you report a large number of patients as being node-negative but no patient reported as being nodal status unknown (NX). For how many patients do we have no information about the lymph nodes, and how do you think that skews the results?
Finally, we are completely missing radioactive iodine therapy information for about two-thirds of the patients, and there is no information on thyroid-stimulating hormone (TSH) suppression, which are the second and third legs of thyroid cancer therapy. How do you think those gaps have affected the results that you have reported?
The second question deals with the robustness of the follow-up. You include a large number of patients, but no life table summaries associated with the survival curves. There must be some decrease in at-risk patients over time. How many patients do you actually have in your follow-up and how do you think that follow-up drop-off has skewed the results?
Finally, I have a comment. In the manuscript, you note that the application of total thyroidectomy around the country could lead to an increase in the complication rates and that public policy should consider this as a part of the guideline recommendations. A total thyroidectomy is the exact sum of 2 thyroid lobectomies, so I would submit if one cannot perform a safe total thyroidectomy, then one should not perform a lobectomy either. I believe that we should clarify this issue to avoid sloppy thinking about reduced complication rates.
Finally, I want to congratulate you again. I think this will clearly be a big part of the discussion regarding the upcoming guidelines for the American Thyroid Association and NCCN as we can finally confidently recommend total thyroidectomy for tumors over 1 centimeter.
Dr. Cord Sturgeon (Chicago, Illinois): The first question concerns the gaps in the NCDB and how might they affect the results. I think there are a number of points that I should highlight.
First, we have no data whatsoever on TSH suppression for any of these patients. That information is not reported in the database. Clearly, that is 1 of the 3 components that we all use for thyroid cancer management: surgery, radioiodine, and then TSH suppression. We do not know the effect of TSH suppression, the degree of TSH suppression, or in fact, if patients even experienced TSH suppression. It is hard to predict the potential bias because we do not know the different effects in each group.
The second question concerns radioiodine administration. As you point out, administration rates are only in the mid-50s in our study. Other studies have shown that radioiodine is administered to around 70% of PTC patients. We think it is probably underreported in this database, just as chemotherapy treatments are underreported in many of these cancer databases. The exact dose of radioiodine timing and frequency of administration is not clear in this study, and it is hard to predict what effect might result 1 way or the other between the 2 groups.
Furthermore, there is no data in this study regarding histopathologic subtypes of papillary thyroid cancer, such as tall cell variant, that might lead to a worse prognosis. We did not determine if these patients had those histologies or not, and that may have effects as well on survival. We have no data on genetics, like BRAF, which we are beginning to understand are very important in this disease as well.
As for node disease, I can tell you with confidence that when we report 34% positive nodes in our thyroidectomy group, those are only patients who had nodes examined, not 34% of all patients including the NX. Roughly 10,000 patients were excluded from that evaluation because we did not have any nodal data.
The other question, how many of these patients received a lobectomy and then maybe went elsewhere and got a completion thyroidectomy and are really total thyroidectomy patients? For the surgery these patients received, we are quite confident that we coded them correctly. For example, there are patients who had a lobectomy at a hospital that reports data to the NCDB and then went to a second hospital that does not contribute to the NCDB and had a completion thyroidectomy. The registrars at the NCDB hospitals are trained to seek out that specific information and code it appropriately. Those patients who received that type of treatment (completion thyroidectomy) are coded as total thyroidectomy in this database.
The next question asked how many patients are represented in the follow-up over time. Of course, over time, patients get censored in this database. At about 5 years of follow-up we still have 66% of our cohort uncensored, alive, and involved in the study, and at ten years, we have about 21% of the patients in this study, roughly 11,000 patients. The results reported at ten years are based on approximately 11,000 individuals with papillary thyroid cancer.
As for the final comment about complication rates, certainly I think that if you look at what Dr. Bilimoria presented, that approximately 12% of patients with PTC larger than 1 cm are receiving thyroid lobectomy, this is the difference that we can potentially make. That group of patients could get a more complete operation and perhaps have a better survival and lower recurrence.
Those would also be the patients exposed to the potential risk of bilateral recurrent laryngeal nerve injury or perhaps permanent hypoparathyroidism. Those risks should be very small in the hands of experienced surgeons. In the United States, the majority of thyroid surgery is not done by experienced surgeons who focus only on thyroidectomy, so that is a major consideration when we promote more extensive surgery. But we think that the overall risk should be quite small and the benefits are merited.
Dr. Thomas R. Russell (Chicago, Illinois): As you pointed out, we have over 20 million cases in the Cancer Database and a large number in the Trauma Database. And I do believe in this new era of value based purchasing that these databases, which were set up years ago by very thoughtful forward-looking surgeons, will give us lots of information about how to do things. So, I think there is a wealth of opportunities for residents or people who want to do clinical health services research to tap into these databases at the college.
I have 2 brief questions about the ease of obtaining this data. How much difficulty is there with the data, given the time frame from when it was first collected in 1988 to the present, and how easy is it to capture the information? Secondly, do you think this study will lead to any changes in the AJCC manual that will be coming out in 2009 with respect to the staging of thyroid cancer?
Dr. Cord Sturgeon (Chicago, Illinois): Certainly, the accumulation of 20 million patients in this database represents a huge amount of work by a large number of individuals, and it has captured, in our estimation, 88% of all thyroid cancers in the United States in the most recent timeframe. Internal reviews suggest that we are capturing the correct operation, we are not duplicating these patients, and these are accurate points of data. But, it does not come easily.
The second question was what effect might this have on the AJCC Seventh Edition that is coming out. I think that is a very interesting question, because what Dr. Bilimoria showed you today is that those tumors that are less than a centimeter in size behave differently than those tumors that are 1 to 2 centimeters in size even correcting for age, radioiodine administration, and nodal status. It does not matter whether you perform a lobectomy or a total thyroidectomy for the small microcarcinomas. But for those tumors that are 1 to 2 centimeters, there is a survival advantage, and there is a lower recurrence rate for the more extensive bilateral operation.
In the current AJCC staging manual, they lumped together those 3, 4 and 5 millimeter microcarcinomas with the 1.7, 1.8, and 1.9 centimeter tumors. Perhaps 1 conclusion from this study is that there may be a different biology between those two, so this study may have some impact on the way we think about T1 versus T2.
Dr. Richard A. Prinz (Chicago, Illinois): I would like to go back to a statement you made that most thyroid surgery is not done by experienced thyroid surgeons. A corollary of that is that the pathologists that examined those thyroidectomy specimens are not experienced pathologists in thyroid disease. You mentioned that tumors were not subtyped. Are you sure that all of these are actually papillary tumors?
Secondly, you have just given us a hazards ratio showing improvement in survival and less recurrence with total thyroidectomy. Although I favor total thyroidectomy, there is obviously a cost to this. Do you have any raw survival data that we can compare with complications? An important thing to note is that we actually benefit only a small number of patients. Could we be buying more complications for a very small increase in survival?
Dr. Cord Sturgeon (Chicago, Illinois): I do not have the exact data that you are looking for confirming that this is papillary and not something else. We have confidence in all the histologies in the database.
The second question regarding raw survival data and the take-home message in terms of the advantage that you offer the patient versus the potential complication? We think that you can achieve an approximately 2% survival advantage over time. You have to compare that to what you think the complication rate is. In the hands of experienced surgeons like you, the risk of something like a recurrent nerve injury or hypoparathyroidism is probably around 0.4%. It is certainly less than 1%. When we think about the overall advantages that we can offer patients by expanding the use of total thyroidectomy, we must compare those 2 numbers. There is roughly a 2% survival benefit for the additional extent of operation (total thyroidectomy), and then the trade-off is the complication rate, which should always be less than 1%, I would say.
Dr. William C. Wood (Atlanta, Georgia): I appreciate the amount of work that has gone into such a massive analysis, and I appreciate the fact that you didn’t come down to a single conclusion. In oncology, we are trying to use large numbers to get information, but then to tailor the treatment to the individual. And your point about sub-centimeter tumors is very helpful.
Many of the large single institution series over the last 30 years have suggested that there are also biological differences poorly understood in pre-menopausal women with papillary carcinoma who fare better than older people and males. Did you see any difference in total thyroidectomy versus lobectomy in pre-menopausal women?
Dr. Cord Sturgeon (Chicago, Illinois): That is an excellent question. We have not looked at that issue at all, but I think it may be in a many people’s minds because gender, and age in particular, has been a focus as strong predictors of overall survival. But we have not looked at pre-menopausal women specifically. That is certainly something we can consider looking into now.
Footnotes
Supported by the American College of Surgeons, Clinical Scholars in Residence program and a Research Grant from the Department of Surgery, Feinberg School of Medicine, Northwestern University (to K.Y.B.).
Reprints: Cord Sturgeon, MD, Northwestern Memorial Hospital, Department of Surgery, 675 N. St. Clair St., Galter 10-105, Chicago, IL 60611. E-mail: csturgeo@nmh.org.
REFERENCES
- 1.American Cancer Society. Cancer facts and figures. Available at: www.cancer.org/docroot/STT/content/STT_1x_Cancer_Facts_Figures_2007.asp. Accessed January 21, 2007.
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson NC, Button J, Solorzano CC. Thyroid cancer: is the incidence still increasing? Ann Surg Oncol. 2004;11:1093–1097. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL. Managing small thyroid cancers. JAMA. 2006;295:2179–2182. [DOI] [PubMed] [Google Scholar]
- 5.Kebebew E, Clark OH. Differentiated thyroid cancer: “complete” rational approach. World J Surg. 2000;24:942–951. [DOI] [PubMed] [Google Scholar]
- 6.Udelsman R, Shaha AR. Is total thyroidectomy the best possible surgical management for well-differentiated thyroid cancer. Lancet Oncol. 2005;6:529–531. [DOI] [PubMed] [Google Scholar]
- 7.Jossart GH, Clark OH. Well-differentiated thyroid cancer. Curr Probl Surg. 1994;31:933–1012. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network (NCCN). Available at: www.nccn.org. Accessed December 15, 2007.
- 9.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. [DOI] [PubMed] [Google Scholar]
- 10.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 11.Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol. 2005;12:81–89. [DOI] [PubMed] [Google Scholar]
- 12.Shaha AR, Shah JP, Loree TR. Low-risk differentiated thyroid cancer: the need for selective treatment. Ann Surg Oncol. 1997;4:328–333. [DOI] [PubMed] [Google Scholar]
- 13.Clark OH. Total thyroidectomy: the treatment of choice for patients with differentiated thyroid cancer. Ann Surg. 1982;196:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udelsman R, Lakatos E, Ladenson P. Optimal surgery for papillary thyroid carcinoma. World J Surg. 1996;20:88–93. [DOI] [PubMed] [Google Scholar]
- 15.Winchester DP, Stewart AK, Bura C, et al. The national cancer data base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85:1–3. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. Cancer facts and figures. Available at: www.cancer.org/docroot/stt/stt_0.asp. Accessed December 15, 2006.
- 17.World Health Organization. International Classification of Disease for Oncology. 2nd ed. Geneva: World Health Organization; 1990. [Google Scholar]
- 18.Standards of the Commission on Cancer. Vol II: Registry Operations and Data Standards. Chicago: Commission on Cancer; 1998. [Google Scholar]
- 19.Facility Oncology Registry Data Standards. Chicago: Commission on Cancer; 2004. [Google Scholar]
- 20.DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–424. [DOI] [PubMed] [Google Scholar]
- 21.United States Census Bureau. Census 2000. Available at: www.census.gov/main/www/cen2000.html. Accessed January 21, 2007.
- 22.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 23.Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204–209. [DOI] [PubMed] [Google Scholar]
- 24.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. thyroid cancer cooperative group. Eur J Cancer. 1979;15:1033–1041. [DOI] [PubMed] [Google Scholar]
- 25.Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–1095. [PubMed] [Google Scholar]
- 26.Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103:2269–2273. [DOI] [PubMed] [Google Scholar]
- 27.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. [DOI] [PubMed] [Google Scholar]
- 28.Samaan NA, Maheshwari YK, Nader S, et al. Impact of therapy for differentiated carcinoma of the thyroid: an analysis of 706 cases. J Clin Endocrinol Metab. 1983;56:1131–1138. [DOI] [PubMed] [Google Scholar]
- 29.Wanebo H, Coburn M, Teates D, et al. Total thyroidectomy does not enhance disease control or survival even in high-risk patients with differentiated thyroid cancer. Ann Surg. 1998;227:912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haigh PI, Urbach DR, Rotstein LE. AMES prognostic index and extent of thyroidectomy for well-differentiated thyroid cancer in the United States. Surgery. 2004;136:609–616. [DOI] [PubMed] [Google Scholar]
- 31.Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–1242. [DOI] [PubMed] [Google Scholar]
- 32.Thyroid Carcinoma Taskforce. AACE/AAES Medical/Surgical Guidelines for Clinical Practice: Management of Thyroid Carcinoma. 2001.
- 33.Passler C, Prager G, Scheuba C, et al. Application of staging systems for differentiated thyroid carcinoma in an endemic goiter region with iodine substitution. Ann Surg. 2003;237:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. Chicago, IL: Springer; 2002. [Google Scholar]
- 35.Shaha AR, Loree TR, Shah JP. Intermediate-risk group for differentiated carcinoma of thyroid. Surgery. 1994;116:1036–1040; discussion 1040–1041. [PubMed]
- 36.Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyrois carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. [DOI] [PubMed] [Google Scholar]
- 37.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–1300. [DOI] [PubMed] [Google Scholar]
- 38.Cress RD, Zaslavsky AM, West DW, et al. Completeness of information on adjuvant therapies for colorectal cancer in population-based cancer registries. Med Care. 2003;41:1006–1012. [DOI] [PubMed] [Google Scholar]
- 39.Du XL, Key CR, Dickie L, et al. Information on chemotherapy and hormone therapy from tumor registry had moderate agreement with chart reviews. J Clin Epidemiol. 2006;59:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(8 suppl):IV-49–54. [DOI] [PubMed] [Google Scholar]
- 41.Cooper GS, Virnig B, Klabunde CN, et al. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40(8 suppl):IV-43–48. [DOI] [PubMed] [Google Scholar]
- 42.Commission on Cancer. Approvals categories. Available at: www.facs.org/cancer/coc/categories.html. Accessed December 17, 2006.
- 43.Percy C, Stanek E III, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sosa JA, Bowman HM, Tielsch JM, et al. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]