Abstract
Objectives:
Catheter-based revascularization has emerged as an alternative to surgical bypass for lower extremity vascular disease and is a frequently used tool in the armamentarium of the vascular surgeon. In this study we report contemporary outcomes of 1000 percutaneous infra-inguinal interventions performed by a single vascular surgery division.
Methods:
We evaluated a prospectively maintained database of 1000 consecutive percutaneous infra-inguinal interventions between 2001 and 2006 performed for claudication (46.3%) or limb-threatening ischemia (52.7%; rest pain in 27.7% and tissue loss in 72.3%). Treatments included angioplasty with or without stenting, laser angioplasty, and atherectomy of the femoral, popliteal, and tibial vessels.
Results:
Mean age was 71.4 years and 57.3% were male; comorbidities included hypertension (84%), coronary artery disease (51%), diabetes (58%), tobacco use (52%), and chronic renal insufficiency (39%). Overall 30-day mortality was 0.5%. Two-year primary and secondary patencies and rate of amputation were 62.4%, 79.3%, and 0.5%, respectively, for patients with claudication. Two-year primary and secondary patencies and limb salvage rates were 37.4%, 55.4%, and 79.3% for patients with limb-threatening ischemia. By multivariable Cox PH modeling, limb-threat as procedural indication (P < 0.0001), diabetes (P = 0.003), hypercholesterolemia (P = 0.001), coronary artery disease (P = 0.047), and Transatlantic Inter-Society Consensus D lesion complexity (P = 0.050) were independent predictors of recurrent disease. For patients that developed recurrent disease, 7.5% required no further intervention, 60.3% underwent successful percutaneous reintervention, 11.7% underwent bypass and 20.5% underwent amputation. Patency rates were identical for the initial procedure and subsequent reinterventions (P = 0.97).
Conclusion:
Percutaneous therapy for peripheral vascular disease is associated with minimal mortality and can achieve 2-year secondary patency rates of nearly 80% in patients with claudication. Although patency is diminished in patients with limb-threat, limb-salvage rates remain reasonable at close to 80% at 2 years. Percutaneous infra-inguinal revascularization carries a low risk of morbidity and mortality, and should be considered first-line therapy in patients with chronic lower extremity ischemia.
Catheter-based revascularization has emerged as an alternative to surgical bypass for lower extremity vascular disease. This publication reports the outcomes of 1000 consecutive percutaneous infra-inguinal interventions for occlusive disease and concludes that percutaneous intervention is associated with minimal mortality, can achieve 2-year secondary patency rates of nearly 80% in claudicants, and 2-year limb-salvage rates of close to 80% in patients with limb-threat.
Peripheral vascular disease manifesting as chronic lower extremity ischemia is responsible for over 400,000 hospital admissions per year in the United States alone and is a marker for significant cardiovascular morbidity.1–3 The clinical presentation can range from intermittent claudication to limb-threatening ischemia. Because of the low risk of limb loss in patients with intermittent claudication, these patients have traditionally been treated with conservative measures, including lifestyle modification, pharmacotherapy, and exercise programs, while reserving surgical intervention for those with severe disabling symptoms.4–6 In contrast, patients with tissue loss or gangrene are at heightened risk of limb loss without intervention, and surgical intervention is usually imperative if amputation is to be avoided.5,7–9
Until recently, open surgical bypass has been the mainstay of therapy for patients requiring intervention for chronic lower extremity vascular disease. There are a plethora of reports that demonstrate that infra-inguinal bypass can be performed with excellent long-term patency and limb-salvage rates.10–12 More recently the emergence of percutaneous techniques for infra-inguinal revascularization has increased the armamentarium of vascular surgeons and allowed for alternative minimally invasive treatments for both patients with claudication and limb-threatening disease.13–19 Balloon angioplasty and stenting, originally confined to the iliac vessels, has now been successfully used to treat infra-inguinal lesions. The morbidity and mortality associated with endoluminal therapy has been found to be less than that reported with traditional open surgical bypass.18–20 Furthermore, an increasing number of techniques for percutaneous therapy have become available, including laser angioplasty, cryoplasty and excisional atherectomy, thereby expanding the extent of and type of lesions amenable to percutaneous treatment.21–23 The less invasive nature of percutaneous intervention relative to open surgical bypass makes it a more acceptable option for patients with lower extremity vascular disease. All of these advantages make percutaneous intervention an ideal therapy for the high-risk cohort of patients that develop peripheral vascular disease.
Although percutaneous infra-inguinal intervention is associated with lower morbidity and mortality, there are concerns regarding the long-term durability of these procedures. Furthermore, it is unclear whether percutaneous intervention will be equally suited for both claudicants and patients with limb-threatening ischemia, as the disease patterns can be quite different in these 2 patient populations. Moreover, it is not defined which patient cohorts respond more or less favorably to these therapies. To address these issues, we report in this analysis the contemporary outcomes of 1000 percutaneous interventions performed by surgeons for lower extremity vascular disease.
PATIENTS AND METHODS
Patients
Between the years 2001 and 2006, 1000 consecutive percutaneous infra-inguinal lower extremity revascularization procedures for chronic lower extremity ischemia were identified and entered into a prospectively maintained database. Indications for intervention included debilitating claudication (defined as severely impairing lifestyle and ranging from 0.5 to 4 blocks) or critical limb ischemia (defined as rest pain, tissue loss or ulceration). Patients were excluded from analysis if they presented with acute lower extremity ischemia.
Demographic information, comorbidities, and details of perioperative events including complications and length of hospital stay were determined by review of patient charts, operative reports and pre- and postoperative studies. Operative reports and angiograms were reviewed to determine lesion type (stenosis or occlusion), severity [Transatlantic Inter-Society Consensus (TASC) classification], location (femoral, popliteal or tibial) and length.44 Postoperatively, patients were followed by physical examination to assess clinical outcome, pulses, ankle:brachial indices, and arterial duplex examination of the treated region. Results of pre- and postoperative noninvasive flow studies were determined by reviewing the divisional vascular laboratory database.
Procedures and Imaging
All procedures were performed by members of the Division of Vascular Surgery. Percutaneous interventions included angioplasty with or without stent placement, cryoplasty, (generally reserved for restenotic lesions), laser angioplasty, and excisional atherectomy. Access for the majority of interventions was via the contralateral femoral artery (69.3%), whereas an ipsilateral antegrade approach was generally employed when isolated tibial disease was anticipated (31.7%). Procedures were performed either with a portable imaging fluoroscopic C-arm (OEC 9800; GE Medical Systems, Milwaukee, WI) or in an angio-equipped operating room using fixed fluoroscopic equipment (Siemens, Munich, Germany) both of which were available on each campus of New York Presbyterian Hospital. Iodinated contrast was used in patients with normal creatinine, and gadolinium or combination gadolinium plus iodinated contrast was used in patients with creatinine level greater that 1.3 mg/dL. The majority of procedures were performed under local anesthesia with intravenous sedation.
Technique
Selective angiography was performed to localize lesions and allow planning of interventions. For all interventions, 5- to 8-Fr sheaths were used. Lesions were crossed with either a luminal or subintimal technique using hydrophilic guide wires (0.035, 0.018, or 0.014 inch). Wires were supported using 4-Fr or 5-Fr angled glide catheters (Angiodynamics, Queensbury, NY) or Quick Cross catheters (Spectranetics, Colorado Springs, CO). Reentry into the luminal space beyond the lesion was confirmed by angiography before further intervention. Balloon angioplasty was performed with appropriately sized noncompliant balloons with inflation times ranging from 60 to 180 seconds at 6 to 15 ATM of pressure. Stenting was performed selectively for >30% residual stenosis or flow limiting dissections. Excisional atherectomy was used as an alternative to balloon angioplasty in 292 (18.1%) of treated vessel locations, most commonly in the popliteal and tibial circulation. The Silverhawk atherectomy device (Fox Hollow Industries, Redwood City, CA), which required a 0.014 inch wire system, was used for all atherectomies. Prior postatherectomy adjunctive procedures including angioplasty or angioplasty/stenting were required in 40.9% of cases either to allow initial passage of the atherectomy device or to treat residual disease after atherectomy. Completion angiography with evaluation of the distal runoff was performed after interventions.
Anticoagulation
Patients were systemically anticoagulated with intravenous heparin (100 units/kg) after placement of a Balkin sheath (Cook, Bloomington, IN) for contralateral procedures or before crossing a critical lesion during ipsilateral procedures. Activating clotting time was maintained above 250 seconds for femoropopliteal procedures and above 300 seconds for tibial interventions. Aspirin was administered to all patients postoperatively unless contraindicated. For patients undergoing stent placement or atherectomy, a loading dose of Plavix 450 mg (Sanofi-Aventis, Bridgewater, NJ) was administered in the post-anesthesia care unit, followed by 75 mg/d for 30 days.
Endpoints
Patients were evaluated postoperatively and then at 6-month intervals by physical examination (pulses, wound healing, presence or absence of claudication or rest pain) and by vascular laboratory exam (ankle:brachial indices and arterial duplex ultrasound). Patency was determined primarily by arterial duplex of the treated vessel and secondarily by ankle:brachial indexes and clinical parameters. Loss of patency on arterial duplex was defined as the presence of an occlusion or a restenosis associated with a velocity ratio of greater than 4:1 (relative to the segment proximal to the treated region). Clinical outcomes included recurrence of claudication, failure of wound healing or the need for major amputation. Hemodynamic failure was defined as a decrease in highest postoperative ankle:brachial index by >0.2 during follow up. With each procedure, an average of 1.6 lesions were treated, and during follow-up a patient was considered to lose patency if restenosis or occlusion was detected in any of the lesions treated. Thus, the analysis was performed by intervention (ie, limb) rather than lesion treated.
Statistical Analysis
Demographic and comorbidity data was reported as mean ± standard deviation or as a percentage by patient (rather than by limb or intervention), whereas all treatment information, complications, patency rates, and other outcomes were reported by intervention. Univariate analysis of dichotomous variables between subgroups was performed by Fisher exact test t test. Primary and secondary patency and limb salvage rates were assessed using Kaplan-Meier survival curves, and subgroups were compared by log-rank analysis to determine factors predictive of treatment failure. Multivariable analysis using Cox-PH modeling was performed to assess factors that were significant on univariate analysis.
RESULTS
Patients
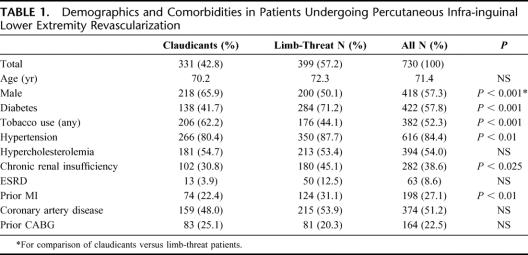
Data were analyzed from 1000 consecutive interventions in 730 patients during the years 2001–2006. This included 856 primary interventions (on 856 limbs) and 144 reinterventions after loss of primary patency. Claudication was the indication in 46.3% of interventions and in 52.7% the indication was limb-threatening ischemia. Limb-threat patients were further divided into those with rest pain (27.7%) and gangrene or tissue loss (72.3%). Mean age was 71.4 ± 11 years (range, 34–98 years), and 57.3% of patients were male. Comorbid medical conditions included diabetes in 57.8%, chronic renal insufficiency in 38.6%, end stage renal disease/hemodialysis in 8.6%, hypertension in 84.4%, a history of tobacco use in 52.3%, hypercholesterolemia in 54.0%, coronary artery disease (CAD) in 51.2%, and prior myocardial infarction (MI) in 27.1%. Patients with limb-threat were significantly more likely to be female, diabetic, hypertensive, hypercholesterolemic or have a history of chronic renal insufficiency (Table 1).
TABLE 1. Demographics and Comorbidities in Patients Undergoing Percutaneous Infra-inguinal Lower Extremity Revascularization
Treatment and Lesion Characteristics
The 1000 interventions included treatment of a total of 1612 lesions. These were categorized by location (Table 2); 721 were femoral lesions (44.7% of treated vessels), 551 were popliteal (34.2%) and 340 were tibial (21.1%). The division between the distal superficial femoral artery and proximal popliteal artery was defined at Hunter’s canal. Lesion distribution differed significantly with each indication. In patients with claudication, a higher proportion of the lesions were found in the femoral location (53.5% in claudication vs. 38.1% in limb-threat, P < 0.001). Conversely, in patients with limb-threat, a higher proportion of lesions were distributed in the tibial location (29.2% in limb-threat vs. 10.4% in claudication, P < 0.001, Table 2).
TABLE 2. Distribution of Treated Lesions in Claudicants and Limb-Threat Patients
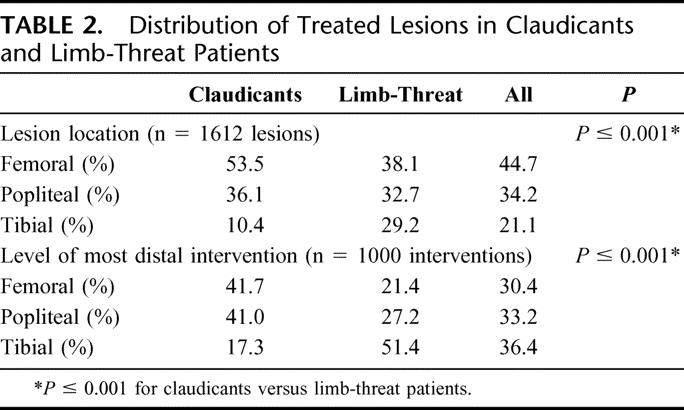
When examining the level of the most distal intervention (femoral, popliteal, or tibial) in each of the 1000 interventions, we found that this factor differed significantly between interventions performed for limb-threat compared with those performed for claudication. Interventions for limb-threat included tibial interventions in 51.4%, compared with only 17.3% of interventions for claudication (P < 0.001, Table 2).
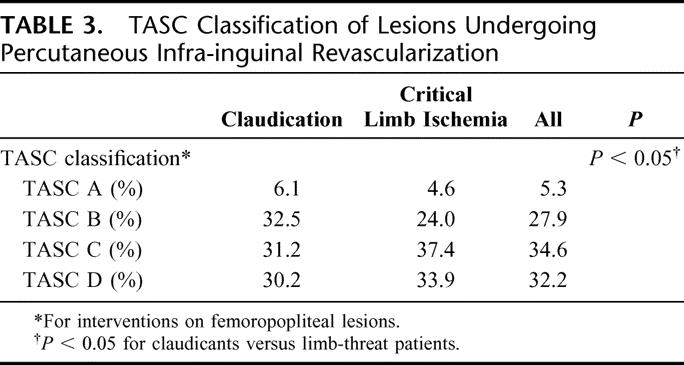
Patients with limb-threat also had a higher number of treated levels (femoral, popliteal, or tibial) per intervention (1.69 vs. 1.48 for claudication, P < 0.001) and a higher proportion of multilevel interventions (52.0% vs. 45.6% for claudication, P < 0.05). Review of lesion characteristics as graded by TASC classification revealed that the majority of patients had predominately moderate to advanced disease as shown in Table 3. There were few TASC A lesions encountered (<6% of all interventions), and a much higher proportion of TASC B-D lesions. Lesions with higher TASC grades were significantly more likely to occur in patients with limb-threatening ischemia (P < 0.05, Table 3). Reduced tibial outflow (<3-vessel runoff) was a prevalent finding, discovered in 82.0% of all interventions and found with significantly increased prevalence in patients with limb-threatening ischemia compared with claudicants (88.1% vs. 67.8%, P < 0.001).
TABLE 3. TASC Classification of Lesions Undergoing Percutaneous Infra-inguinal Revascularization
Outcomes
Perioperative 30-day mortality was 0.5%. Complications included groin hematoma in 5.0% (requiring operative exploration in 0.8%), and pseudoaneurysm formation 3.4% (all treated by thrombin injection). Renal dysfunction occurred in 2.0%, resulting in the need for hemodialysis in 0.4%. Of the 4 patients requiring hemodialysis, the baseline creatinine was >4.0 mg/dL in both patients that became dialysis-dependent and between 2.6 and 3.4 mg/dL in the 2 patients who required transient dialysis. The indication for intervention in each of these 4 patients was limb-threatening ischemia.
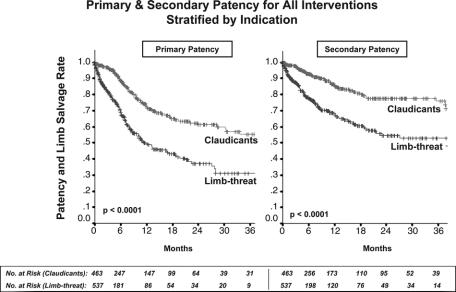
Primary and secondary patency rates for all interventions and limb-salvage rates for patients with limb-threat are illustrated by Kaplan-Meier curves and compared by log-rank analysis (Fig. 1). For all interventions, 12, 24, and 30-month primary patency rates were 61% ± 2%, 50% ± 3%, and 48% ± 1%, whereas secondary patency rates were significantly higher at 76.7% ± 2%, 68% ± 2%, and 66% ± 2% (P < 0.001). Limb-salvage rates (in patients with limb-threatening conditions) were 82% ± 2%, 79% ± 3%, and 79% ± 3%. Mean follow-up for all patients was 9.9 months.
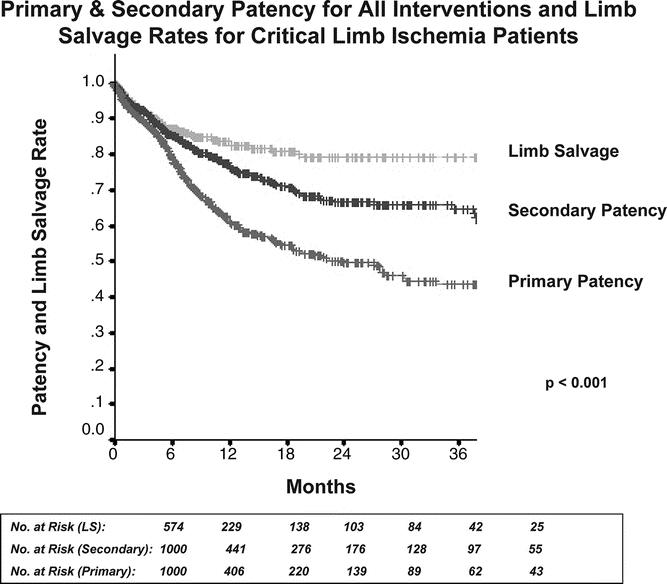
FIGURE 1. Primary and secondary patency rates for all interventions and limb-salvage rates for patients with limb-threatening ischemia (rest pain or tissue loss). Secondary patency was significantly higher than primary patency, reaching 66% at 2.5 years. Overall limb-salvage rate in patients with limb-threat was 79% at 2.5 years.
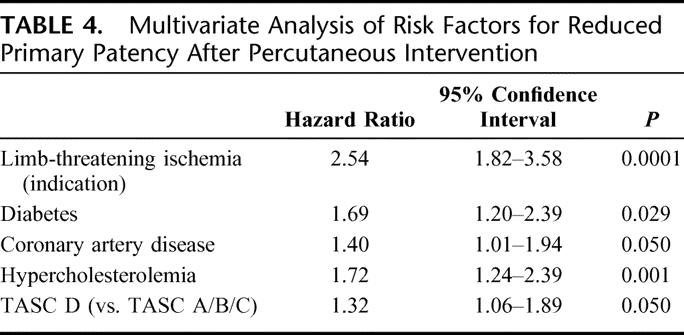
Log-rank analysis of patency rates stratified by indication for intervention demonstrated that patients with limb-threat were much more likely to achieve reduced primary and secondary patency than claudicants (P < 0.0001, Fig. 2). For limb-threat patients compared with claudicants, 24-month primary patencies were 37% ± 4% versus 62% ± 4% (P < 0.0001) and secondary patencies were 55% ± 4% versus 79% ± 3% (P < 0.0001). Furthermore, using multivariable Cox-PH modeling, limb-threat as an indication for intervention proved to be the single most important predictor of recurrent stenosis or occlusion (P < 0.0001, Table 4).
FIGURE 2. Primary and secondary patency rates for all interventions stratified by indication for procedure. Claudicants demonstrated significantly higher primary and secondary patency than patients with limb-threat.
TABLE 4. Multivariate Analysis of Risk Factors for Reduced Primary Patency After Percutaneous Intervention
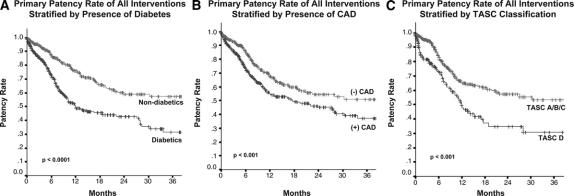
Additional predictors on univariate analysis of loss of primary patency included diabetes, CAD, and increasing TASC classification (TASC A/B/C vs. D). For example, 24-month secondary patencies for patients with diabetes were 59% ± 4% compared with 76% ± 3% for nondiabetics (P < 0.0001, Fig. 3A). Twenty-four month secondary patencies for patients with CAD were 61% ± 3% compared with 73%± 3% for patients without a history of CAD (P < 0.0005, Fig. 3B). Interventions for TASC D (complex) lesions had a 24-month secondary patency rate of 55% ± 2% compared with 70% ± 1% for those for TASC A/B/C lesions (P < 0.01, Fig. 3C).
FIGURE 3. Primary patency rates for all interventions stratified by risk factors for patency loss on univariate analysis including (A) diabetes, (B) coronary artery disease, and (C) lesions of increasing severity. Each of these factors was associated with decreased primary patency.
Mutlivariate Cox-PH modeling risk factors confirmed limb-threat as the procedural indication (P < 0.0001), diabetes (P = 0.029), CAD (P = 0.050), and TASC D lesions (P = 0.050), as well as hypercholesterolemia (P = 0.001), as significant independent predictors of reduced primary patency (Table 4). Gender, hypertension, history of tobacco use, prior MI, prior coronary artery bypass graft, chronic renal insufficiency, and end-stage renal disease/hemodialysis were not found to be predictive of loss of patency.
The effect of treatment modality on outcome was analyzed by treated region (femoral, popliteal, or tibial). In the femoral and popliteal regions there was a trend toward improved outcome with angioplasty alone and a poorer outcome with atherectomy, although this was not statistically significant (P = 0.06 femoral, P = 0.17 popliteal). Of note this was not a randomized study and patients treated with angioplasty alone may well have had less severe disease. In the tibial location, all modalities appeared equally effective, but 2-year primary patency rates were quite low, ranging from 34% to 38% (P = 0.87). Secondary patency rates for tibial lesions were much higher, and were also similar between modalities ranging from 61% to 72% (P = 0.38).
The overall rate of percutaneous reintervention over the follow-up period was 16.8%; this number was similar for claudicants (17.0%) and patients with limb-threat (16.6%). Interestingly, the patency rate after reintervention was equivalent to that of the primary intervention (P = 0.26, Fig. 4). After failure of percutaneous therapy, 17 patients with limb-threat (3.6% of initial interventions or 11.4% of those who had a percutaneous failure) underwent successful surgical bypass for limb-salvage, and 47 patients (10.4% of initial interventions, 31.5% of percutaneous failures) underwent major amputation. Eleven patients (2.8% initial interventions, 12.2% of percutaneous failures) with claudication underwent surgical bypass for relief of symptoms after the failure of percutaneous therapy. Two patients (0.5%) with claudication ultimately underwent amputation after progression of disease.
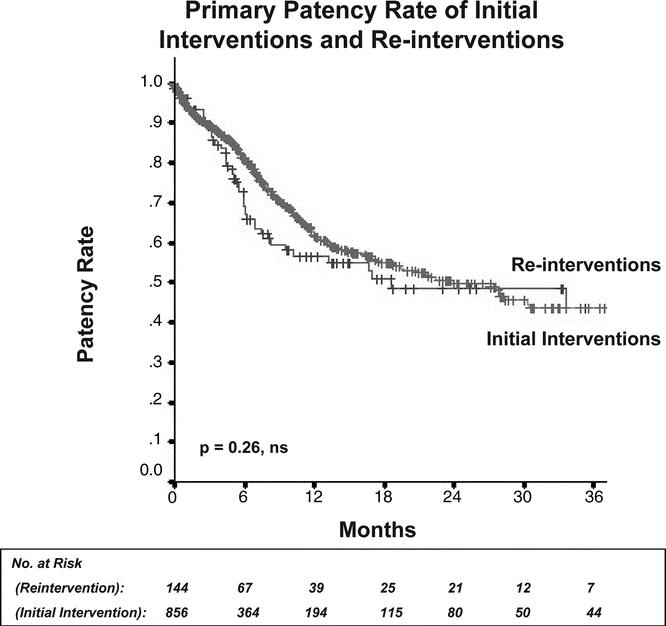
FIGURE 4. Primary patency rate of initial interventions compared with reinterventions. Reinterventions proved as durable as primary interventions.
DISCUSSION
Open surgical bypass has been used after failure of conservative therapy with excellent outcomes for patients with severe symptoms of claudication.24,25 Furthermore, surgical bypass for many years has been considered the gold standard for the treatment of limb-threatening ischemia.10–12,35 Although 5-year patency rates of 80% are often quoted in many single-institutional series, these excellent results are generally obtained in patients with favorable anatomy and acceptable vein conduit. Interestingly, results from a recently reported multicenter trial of infra-inguinal bypass were not as favorable, with 1-year primary patency rates of 61%.26 When it is necessary to use alternative conduits such as prosthetic graft or cryopreserved vein, 5-year patency rates can be as low as 25%.27 Moreover, because patients with peripheral vascular disease have multiple comorbid medical illnesses, the morbidity and mortality associated with open surgical bypass is not inconsequential. Mortalities range from 2% to 10%, and complication rates in this group can range from 20% to 50%.14,27–30 Even in the absence of significant complications, recovery can be prolonged. In fact, it has been reported that less than 50% of patients return to their preoperative functional status by 6 months.31 The impact of a long surgical procedure and hospitalization, as well as the effect of perioperative complications, upon quality of life should not be underestimated. To some extent improving or maintaining quality of life is the reason for performing the bypass in many of these patients.
Over the last decade, percutaneous intervention for infra-inguinal vascular disease has evolved considerably. There has been a rapid emergence of new technology, which has disseminated to the vascular surgery community, allowing surgeons to percutaneously treat lesions of increasing complexity.32,33 High rates of initial success and reasonable midterm patencies have led a number of investigators to adopt endovascular intervention as the first-line modality in patients with chronic lower extremity disease.16,18,19,34
Percutaneous lower extremity intervention is well suited for both claudicants and patients with limb-threatening disease, albeit for somewhat different reasons. Many surgeons are reluctant to offer patients with claudication a surgical bypass because of the attendant morbidity and mortality that accompanies these procedures, which even in contemporary series can be as high as 10% and 3%, respectively.34 These risks are compounded by the fact that patients with claudication are at a very low risk of long term limb-loss. Percutaneous intervention has offered for many patients an option that is superior to medical therapy, which provides a more acceptable peri-procedural risk and morbidity. In this report, there were no deaths among patients with claudication, and the rate of major complication (access complications requiring operative intervention or acute renal failure) was 1.5%, which compares quite favorably to the outcomes of lower extremity bypass.34 Additionally, patients with claudication are more willing to undergo intervention if discomfort is minimal and if the recovery period is shortened to an overnight hospital stay as is the case with percutaneous intervention. Although we did not measure quality of life in these patients, a recent prospective study demonstrated excellent functional outcomes and good patient satisfaction after percutaneous intervention, particularly in patients with claudication.7
Alternatively, in patients with limb-threatening disease, revascularization is generally mandatory for limb-salvage. However, this patient cohort typically has numerous associated cardiovascular risk factors that increase perioperative risk. The mortality and major morbidity in this series of patients treated for limb-threatening disease with percutaneous interventions was only 1.0% and 4.9%, respectively, compared with usual mortalities and morbidities of 5% and 30% for open surgical bypass.14,27,29 To some extent, it appears that the short-term advantages of minimally invasive vascular therapies are often counterbalanced by diminished durability. Percutaneous therapy for limb-threatening lower extremity vascular disease is no exception, with primary patency rates at 30 months of 31.1%. However, secondary patency rates were more reasonable at 53.2%, and more importantly, limb salvages rates were near 80% at 30 months. It might be argued that durability may be less relevant in this subset of patients that generally have 5-year survival rates of less than 50%. Most of these patients ultimately succumb to cardiovascular disease in other locations. A less risky intervention associated with reduced discomfort and lower morbidity that can maintain limb viability is of tremendous advantage in this morbid group of patients, particularly those in whom life-expectancy is limited.
This report includes procedures performed exclusively by vascular surgeons and represents one of the largest available series of percutaneous infra-inguinal revascularization. Primary patency for all interventions in this series was 61% and 50% at 12 and 24 months, respectively, which compares favorably to other reports of percutaneous lower extremity revascularization, especially considering the relatively large number of tibial interventions (21.1% overall) and high degree of lesion complexity (almost two-thirds were TASC C & D lesions) found in these patients. Prior studies where femoropopliteal interventions were predominant report primary patency rates ranging from 27% to 60%.19,36–39
In contrast to failed surgical bypass grafts, lesions treated by percutaneous intervention that have developed restenosis or occlusion are amenable to reintervention. The overall rate of percutaneous reintervention in our study was 16.8%, and interestingly, the primary patency of these reinterventions did not differ from that of the initial intervention (Fig. 4). The effect of percutaneous reintervention is seen in the secondary patency rate, which was nearly 70% at 24 months for all interventions, a rate that approaches patency rates reported in some series of surgical bypass.26
Our multivariate analysis revealed several factors associated with loss of primary patency. Limb-threat as the procedural indication was the strongest predictor of patency loss. When patients with claudication were analyzed separately from patients with limb-threat, substantial differences in the rate of primary and secondary patency were found. For example, claudicants can anticipate a 24-month secondary patency of nearly 80%, whereas the patency for patients with limb-threat was only 55%. Similar results at 12 months have been described by Kalbaugh and coworkers (primary patency of 78% and 35% for claudicants and limb-threat, respectively) as well as others.7,37,40 Despite the relatively low patency rate in limb-threat patients relative to claudicants, many of these patients were able to heal wounds or minor amputation sites during the period of revascularization, and thus maintain a rate of limb viability of near 80%.16,7,41 The limb salvage rate in this patient group is somewhat lower than the 90% that has been reported in several large series of surgical revascularization. The reasons for this are not entirely clear but likely multifactorial. A number of patients in this series were referred with advanced disease and without surgical alternatives, either because of anatomic constraints (lack of conduit) or prohibitive medical comorbidites. Thus, failure of percutaneous revascularization in these patients was not followed by attempt at surgical bypass. We have previously reported on a series of such patients that are contained within this cohort.20 We were not able to identify in this series any patients where percutaneous intervention led to loss of a surgical bypass option. Thus, it does not appear from our data, that an attempt at percutaneous revascularization eliminates the option of surgical bypass if the percutaneous intervention is unsuccessful.
Although not universal,41 most studies have demonstrated reduced patency rates in patients with diabetes after infra-inguinal percutaneous intervention, a finding that is confirmed by our data.16,42,43 Lesion complexity (including TASC classification) has also previously been associated with failure after percutaneous interventions.34,37,39 Similarly, we found reduced primary and secondary patency with TASC D lesions compared with TASC A, B & C. Other predictors of loss of patency in our study included CAD and hypercholesterolemia, which have been less commonly implicated in prior studies.
The increased utilization of percutaneous techniques for infra-inguinal revascularization has been prompted by recent technological advances. There are a number of different techniques available for treating infra-inguinal disease, including angioplasty alone, angioplasty and stent, cryoplasty, laser angioplasty, and excisional atherectomy. The current study demonstrated no significant differences between treatment modalities in either the femoral, popliteal, or tibial locations. However, the nonrandomized nature of this study likely has led to treatment selection bias. It is possible that when patients with similar disease severity and lesion characteristics are compared prospectively, 1 modality will prove superior to the others. Despite the large number of patients available in this analysis there were insufficient numbers with each technique to perform subset analysis.
CONCLUSIONS
Percutaneous infra-inguinal revascularization has rapidly emerged as an alternative to open surgical bypass in patients with chronic lower extremity ischemia. Two-year secondary patency rates of nearly 80%, which rival those of surgical bypass, are attainable in patients with claudication. Although primary and secondary patency rates were lower for patients presenting with limb-threatening ischemia, long-term durability of revascularization in these patients is of less concern because of their decreased longevity from cardiovascular events. In this patient cohort the reasonable short-term patency rates associated with percutaneous intervention allowed for limb-salvage in approximately 80% of patients. Percutaneous infra-inguinal revascularization carries a low risk of morbidity and mortality, is well accepted by patients, does not prevent ultimate surgical revascularization if necessary and consequently should be considered the first-line therapy for chronic lower extremity ischemia.17,34,36
Discussions
Dr. Gregorio A. Sicard (St. Louis, Missouri): This represents the largest single center series that I am aware of. But, what I think is more important is that it is done by surgeons, and these results are as good as or better than any smaller series reported in the literature by other fields performing these procedures.
Forty-six percent of your patients that were treated were claudicant. Will you clarify if the percutaneous technique was the initial therapeutic approach proposed to these patients? Or was risk factor modification, supervised gradual exercise program, or best medical therapy the first line of therapy? In other words, what was the denominator in your claudication group and in your critical limb ischemia group? If you treated a thousand patients, how many patients were seen that could be classified in those 2 groups?
This series spans over 5 years, during which time the technology of peripheral interventions has advanced significantly. Was there a similar distribution during that 5-year period of the therapeutic approach? Or was there some bias as new technologies came about, better stents, better balloons, which might have modified or changed your results? And do you have, with a thousand patients, enough subgroup analysis ability to give us that information?
I would like to point out that in the critical limb ischemia time (and I think you showed that very well and alluded to that in your presentation) that there is more of a precipitous drop in terms of the failure or the occlusion, and that it occurs much earlier. In the first 6 or 12 months there is a significant drop in that group of patients.
Have you changed the algorithm therapy in your institution? Do you have a good saphenous vein; you have a critical limb ischemia? Or do you still think that because your secondary intervention gives such good results that you should offer this as a first line of therapy for those patients?
Dr. Brian G. DeRubertis (New York, New York): As you pointed out, it is true that these procedures were all done by surgeons. We feel that anyone who is adequately trained and knows how to do these procedures well should have full access to the resources required to do so. Of course our bias is that as surgeons we know when we are putting a bypass-target vessel at risk. We know that if we are unable to re-enter a popliteal artery at the above-knee location, and begin going to the below knee segment, this changes the nature of the subsequent salvage operation. This is certainly 1 reason we feel that surgeons are the most appropriate physicians to become familiar with endovascular techniques and to really embrace this technology.
In terms of risk factor modification or the optimal medical management for these patients prior to intervening upon them for claudication, all of our patients receive recommendations in terms of statin use, blood-pressure control, smoking cessation, and exercise programs. This remains the first line of treatment for claudicants. Some of them will get better with that approach, and some of them, even despite improvement, will still want better symptom relief. Unfortunately, I cannot give you the denominator because many of our patients are referred to us specifically for these procedures and have already gone through that process of optimal medical management.
Technology certainly has changed dramatically over time. We changed our approach based on the change in technology and our familiarity with these techniques. Whereas 5 years ago we were doing many more open surgical bypass graft operations, over time that has become a fall-back or second-line treatment, and percutaneous intervention has become our first-line modality in almost all patients with few exceptions.
In addition to the evolution from open techniques to percutaneous ones, there has been a change in the nature of available percutaneous devices as well. Five years ago we did more primary angioplasties or angioplasty and stent procedures than we do now. Now we are performing more atherectomies to complement the angioplasty and stenting depending on location and the pattern of disease. As I pointed out, the outcomes are not necessarily any different between the different modalities, but our impression is that this may reflect a treatment selection bias. We may be using certain devices for specific patients with more severe disease.
Dr. Ronald M. Fairman (Philadelphia, Pennsylvania): I have 3 questions:
Limb-threatening ischemia was the number 1 predictor of recurrent stenosis or occlusion. Can you explain why this might be the case? Were these patients more likely to have multilevel lesions?
Do you do these procedures as an outpatient and do you use closure devices?
Lastly, your data on tibial interventions suggests that all therapeutic modalities were equally effective with higher secondary patency rates. Given the increased cost associated with some of these newer technologies, what is your primary strategy for intrapopliteal disease? Do you begin with angioplasty and then go on to the more expensive technology?
Dr. Brian G. DeRubertis (New York, New York): To answer your first question regarding the worse outcome in limb-threat patients, I think there are a number of factors. Limb-threat patients in general are more likely to be diabetic, to have hypertension, and to have a number of factors such as tibial interventions or multilevel disease. All of these probably play a role in outcome, as well as a number of factors that we haven’t yet discovered. I think they all contribute to some degree.
We do not currently perform these as outpatient procedures. Any patient who gets an intervention stays overnight in the hospital and thus has a 1-day hospital stay. We began using closure devices routinely although the main benefit of those devices lies in diagnostic procedures where patients can be discharged the same day. There are, however, other benefits in terms of patient discomfort and early ambulation.
Comparing the different modalities we have not been able to demonstrate superiority of 1 modality over another in specific settings, such as in the tibial or popliteal circulations. For example, patency in the tibial region is similar for all modalities with approximately 35% primary patency at 2 years. Therefore, it is difficult to justify a more expensive device until we are sure we are comparing outcomes in patients with equivalent disease severity. Hopefully, as we stratify these patients and their disease severity better we will be able to get that answer.
Dr. Dhiraj M. Shah (Albany, New York): I just want to mention that your experience and ours, and every large series, shows that bypass to the distal artery patency rate is about 10% to 20% higher in limb-salvaged patients. Similarly, the limb-salvage rate is higher, too. Is yours a different group of patients, and the limb-salvage rate is lower than the published data? If this is so for this group of patients when they come for a second procedure, albeit at the same baseline, do they have the same conditions?
Dr. Brian G. DeRubertis (New York, New York): Your question focuses on percutaneous failures, especially in the tibial location, and whether these patients who fail go back to their prior baseline or develop worsened levels of ischemia. While we have certainly seen cases in which a surgical bypass occludes and takes with it some of the outflow bed, we generally have not seen such a phenomenon in percutaneous interventions. A treated lesion can develop restenosis or occlusion without affecting the distal runoff or collateral vessels. Furthermore, we have also found that reintervention on that area of re-stenosis or occlusion has been relatively easy in most settings. These re-interventions result in sustained secondary patency, which is often long enough for a wound to heal and the patient to avoid an amputation.
Dr. Paul J. DiMuzio (Philadelphia, Pennsylvania): If you would, please clarify how your database was maintained in the case of an unsuccessful attempt. For example, if I do an arteriogram and am unsuccessful in crossing a lesion, I bill and record the case simply as an arteriogram. Given this possibility in your record keeping, what do you think was your primary success rate in treating the lesions?
Dr. Brian G. DeRubertis (New York, New York): When we are unsuccessful at crossing a lesion, we count that as a failure of percutaneous therapy. These procedures do not go into the patency curves but they do count as technical failures. Our overall technical success rate is presently 94%. However, it is possible that the actual rate may be closer to the 85%–90% range as some attempted recannalizations may be incorrectly classified as diagnostic studies only, as your question suggests.
Dr. John C. Connolly (Orange, California): I have a question about your use of atherectomy for extensive disease of the superficial femoral artery, which I believe was 15% of your patients. Forty years ago the accepted management was a semiclosed endarterectomy employing either a Wylie or Cannon Ring stripper to remove a core of disease like that produced by your atherectomy device. The other procedure was to open the artery with a long incision and remove all of the disease and apply a long vein patch (Edwards). Neither of these procedures provided patency more than 1–2 years and was discarded. Re-intervention was never tried on these failures and femoral-popliteal bypass became the gold standard.
I have wondered if most vascular surgeons are now unfamiliar with this history. I am sure our moderator today, Dr. Busuttil used the Cannon strippers back when he was a budding vascular surgeon! I would like to know what percent of your superficial femoral artery atherectomy patients required re-intervention and what have the results been?
Dr. Brian G. DeRubertis (New York, New York): While we may be relearning history with some of these devices, there are inherent differences between current atherectomy devices and prior methods of atherectomy.
I think the major difference is the fact that we can re-intervene on these patients with minimal morbidity or mortality, and minimal discomfort to the patient. This is why some patients, when faced with the single operation that might be more durable, will instead opt for 2 or even 3 procedures done under a local anesthetic with a single-day hospital stay.
Footnotes
Reprints: K. Craig Kent, MD, Division of Vascular Surgery, New York Presbyterian Hospital, Weill Medical College of Cornell University, Columbia University, College of Physicians and Surgeons, 525 E. 68th St., Room P-707, New York, NY 10021. E-mail: kckent@med.cornell.edu.
REFERENCES
- 1.Hackam DG. Cardiovascular risk prevention in peripheral artery disease. J Vasc Surg. 2005;41:1070–1073. [DOI] [PubMed] [Google Scholar]
- 2.Yeager RA, Moneta GL, Taylor LM Jr, et al. Surgical management of severe acute lower extremity ischemia. J Vasc Surg. 1992;15:385–391; discussion 392–393. [DOI] [PubMed]
- 3.Hunink MG, Wong JB, Donaldson MC, et al. Revascularization for femoropopliteal disease. A decision and cost-effectiveness analysis. JAMA. 1995;274:165–171. [PubMed] [Google Scholar]
- 4.Garcia LA. Epidemiology and pathophysiology of lower extremity peripheral arterial disease. J Endovasc Ther. 2006;13:II3–II9. [DOI] [PubMed] [Google Scholar]
- 5.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. [DOI] [PubMed] [Google Scholar]
- 6.Schmieder FA, Comerota AJ. Intermittent claudication: magnitude of the problem, patient evaluation, and therapeutic strategies. Am J Cardiol. 2001;87:3D–13D. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SM, Kalbaugh CA, Blackhurst DW, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44:747–755; discussion 755–756. [DOI] [PubMed]
- 8.Lepantalo M, Matzke S. Outcome of unreconstructed chronic critical leg ischaemia. Eur J Vasc Endovasc Surg. 1996;11:153–157. [DOI] [PubMed] [Google Scholar]
- 9.Bertele V, Roncaglioni MC, Pangrazzi J, et al. Clinical outcome and its predictors in 1560 patients with critical leg ischaemia. Chronic Critical Leg Ischaemia Group. Eur J Vasc Endovasc Surg. 1999;18:401–410. [DOI] [PubMed] [Google Scholar]
- 10.Veith FJ, Ascer E, Gupta SK, et al. Tibiotibial vein bypass grafts: a new operation for limb salvage. J Vasc Surg. 1985;2:552–557. [DOI] [PubMed] [Google Scholar]
- 11.Veith FJ, Moss CM, Daly V, et al. New approaches to limb salvage by extended extra-anatomic bypasses and prosthetic reconstructions to foot arteries. Surgery. 1978;84:764–774. [PubMed] [Google Scholar]
- 12.Sayers RD, Raptis S, Berce M, et al. Long-term results of femorotibial bypass with vein or polytetrafluoroethylene. Br J Surg. 1998;85:934–938. [DOI] [PubMed] [Google Scholar]
- 13.Morgan JH III, Wall CE Jr, Christie DB, et al. The results of superficial femoral, popliteal, and tibial artery stenting for peripheral vascular occlusive disease. Am Surg. 2005;71:905–909; discussion 909–910. [PubMed]
- 14.Adam DJ, Beard JD, Cleveland T, et al; BASIL Trial Participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. [DOI] [PubMed] [Google Scholar]
- 15.Eskelinen E, Alback A, Roth WD, et al. Infra-inguinal percutaneous transluminal angioplasty for limb salvage: a retrospective analysis in a single center. Acta Radiol. 2005;46:155–162. [DOI] [PubMed] [Google Scholar]
- 16.Black JH III, LaMuraglia GM, Kwolek CJ, et al. Contemporary results of angioplasty-based infrainguinal percutaneous interventions. J Vasc Surg. 2005;42:932–969. [DOI] [PubMed] [Google Scholar]
- 17.Parsons RE, Suggs WD, Lee JJ, et al. Percutaneous transluminal angioplasty for the treatment of limb threatening ischemia: do the results justify an attempt before bypass grafting? J Vasc Surg. 1998;28:1066–1071. [DOI] [PubMed] [Google Scholar]
- 18.Kudo T, Chandra FA, Kwun WH, et al. Changing pattern of surgical revascularization for critical limb ischemia over 12 years: endovascular vs. open bypass surgery. J Vasc Surg. 2006;44:304–313. [DOI] [PubMed] [Google Scholar]
- 19.Kudo T, Chandra FA, Ahn SS. The effectiveness of percutaneous transluminal angioplasty for the treatment of critical limb ischemia: a 10-year experience. J Vasc Surg. 2005;41:423–435; discussion 435. [DOI] [PubMed]
- 20.Clair DG, Dayal R, Faries PL, et al. Tibial angioplasty as an alternative strategy in patients with limb-threatening ischemia. Ann Vasc Surg. 2005;19:63–68. [DOI] [PubMed] [Google Scholar]
- 21.Laird JR, Biamino G, McNamara T, et al. Cryoplasty for the treatment of femoropopliteal arterial disease: extended follow-up results. J Endovasc Ther. 2006;13(suppl 2):II52–II59. [DOI] [PubMed] [Google Scholar]
- 22.Yancey AE, Minion DJ, Rodriguez C, et al. Peripheral atherectomy in TransAtlantic intersociety consensus type C femoropopliteal lesions for limb salvage. J Vasc Surg. 2006;44:503–508. [DOI] [PubMed] [Google Scholar]
- 23.Laird JR, Zeller T, Gray BH, et al; LACI Investigators. Limb salvage following laser-assisted angioplasty for critical limb ischemia: results of the LACI multicenter trial. J Endovasc Ther. 2006;13:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Klinkert P, Schepers A, Burger DH, et al. Vein versus polytetrafluoroethylene in above-knee femoropopliteal bypass grafting: five-year results of a randomized controlled trial. J Vasc Surg. 2003;37:149–155. [DOI] [PubMed] [Google Scholar]
- 25.Zannetti S, L’Italien GJ, Cambria RP. Functional outcome after surgical treatment for intermittent claudication. J Vasc Surg. 1996;24:65–73. [DOI] [PubMed] [Google Scholar]
- 26.Conte MS, Bandyk DF, Clowes AW, et al; PREVENT III Investigators. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. [DOI] [PubMed] [Google Scholar]
- 27.Klinkert P, van Dijk PJ, Breslau PJ. Polytetrafluoroethylene femorotibial bypass grafting: 5-year patency and limb salvage. Ann Vasc Surg. 2003;17:486–491. [DOI] [PubMed] [Google Scholar]
- 28.Goshima KR, Mills JL Sr, Hughes JD. A new look at outcomes after infrainguinal bypass surgery: traditional reporting standards systematically underestimate the expenditure of effort required to attain limb salvage. J Vasc Surg. 2004;39:330–335. [DOI] [PubMed] [Google Scholar]
- 29.Schepers A, Klinkert P, Vrancken Peeters MP, et al. Complication registration in patients after peripheral arterial bypass surgery. Ann Vasc Surg. 2003;7:198–202. [DOI] [PubMed] [Google Scholar]
- 30.L’Italien GJ, Cambria RP, Cutler BS, et al. Comparative early and late cardiac morbidity among patients requiring different vascular surgery procedures. J Vasc Surg. 1995;21:935–944. [DOI] [PubMed] [Google Scholar]
- 31.Gibbons GW, Burgess AM, Guadagnoli E, et al. Return to well-being and function after infrainguinal revascularization. J Vasc Surg. 1995;21:35–44; discussion 44–45. [DOI] [PubMed]
- 32.Dosluoglu HH, Harris LM, Cherr GS. Recanalization of concomitant iliac artery and common femoral artery occlusions using an antegrade hybrid (open/endovascular) approach. Vasc Endovascular Surg. 2007;41:130–134. [DOI] [PubMed] [Google Scholar]
- 33.Pavkov ML, Lermusiaux P, Bleuet FO, et al. Simultaneous ipsilateral infrainguinal angioplasty and bypass procedures. Vascular. 2007;15:30–34. [DOI] [PubMed] [Google Scholar]
- 34.Conrad MF, Cambria RP, Stone DH, et al. Intermediate results of percutaneous endovascular therapy of femoropopliteal occlusive disease: a contemporary series. J Vasc Surg. 2006;44:762–769. [DOI] [PubMed] [Google Scholar]
- 35.Abbruzzese TA, Havens J, Belkin M, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg. 2004;39:1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molloy KJ, Nasim A, London NJ, et al. Percutaneous transluminal angioplasty in the treatment of critical limb ischemia. J Endovasc Ther. 2003;10:298–303. [DOI] [PubMed] [Google Scholar]
- 37.Surowiec SM, Davies MG, Eberly SW, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2005;41:269–278. [DOI] [PubMed] [Google Scholar]
- 38.Lofberg AM, Karacagil S, Ljungman C, et al. Percutaneous transluminal angioplasty of the femoropopliteal arteries in limbs with chronic critical lower limb ischemia. J Vasc Surg. 2001;34:114–121. [DOI] [PubMed] [Google Scholar]
- 39.Lazaris AM, Salas C, Tsiamis AC, et al. Factors affecting patency of subintimal infrainguinal angioplasty in patients with critical lower limb ischemia. Eur J Vasc Endovasc Surg. 2006;32:668–674. [DOI] [PubMed] [Google Scholar]
- 40.Smith BM, Stechman M, Gibson M, et al. Subintimal angioplasty for superficial femoral artery occlusion: poor patency in critical ischaemia. Ann R Coll Surg Engl. 2005;87:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tefera G, Turnipseed W, Tanke T. Limb salvage angioplasty in poor surgical candidates. Vasc Endovascular Surg. 2003;37:99–104. [DOI] [PubMed] [Google Scholar]
- 42.Clark TW, Groffsky JL, Soulen MC. Predictors of long-term patency after femoropopliteal angioplasty: results from the STAR registry. J Vasc Interv Radiol. 2001;12:923–933. [DOI] [PubMed] [Google Scholar]
- 43.Hewes RC, White RI Jr, Murray RR, et al. Long-term results of superficial femoral artery angioplasty. AJR Am J Roentgenol. 1986;146:1025–1029. [DOI] [PubMed] [Google Scholar]
- 44.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC working group. TransAtlantic Inter-Society Concensus (TASC). J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]