Abstract
Objective:
To examine the prevalence of BRAF mutation among thyroid cancer histologic subtypes and determine the association of BRAF mutation with indicators of poor prognosis for papillary thyroid cancer and patient outcome.
Summary Background Data:
The appropriate extent of surgical treatment, adjuvant therapy and follow-up monitoring for thyroid cancer remains controversial. Advances in the molecular biology of thyroid cancer have helped to identify candidate markers of disease aggressiveness. A commonly found genetic alternation is a point mutation in the BRAF oncogene (BRAF V600E), which is primarily found in papillary thyroid cancer and is associated with more aggressive disease.
Methods:
BRAF V600E mutation status was determined in 347 tumor samples from 314 patients with thyroid cancer (245 with conventional papillary thyroid cancer, 73 with follicular thyroid cancer, and 29 with the follicular variant of papillary thyroid cancer). Univariate and multivariate analyses were performed to determine the association of BRAF V600E with clinicopathologic factors and patient outcome.
Results:
The prevalence of BRAF V600E mutation was higher in conventional papillary thyroid cancer (51.0%) than in follicular variant of papillary thyroid cancer (24.1%) and follicular thyroid cancer (1.4%) (P < 0.0001). In patients with conventional papillary thyroid cancer, BRAF V600E mutation was associated with older age (P = 0.0381), lymph node metastasis (P = 0.0323), distant metastasis (P = 0.045), higher TNM stage (I and II vs. III and IV, P = 0.0389), and recurrent and persistent disease (P = 0.009) with a median follow-up time of 6.0 years. Multivariate analysis showed that BRAF V600E mutation [OR (95% CI) = 4.2 (1.2–14.6)] and lymph node metastasis [OR (95% CI) = 7.75 (2.1–28.5)] were independently associated with recurrent and persistent disease in patients with conventional papillary thyroid cancer.
Conclusions:
BRAF V600E mutation is primarily present in conventional papillary thyroid cancer. It is associated with an aggressive tumor phenotype and higher risk of recurrent and persistent disease in patients with conventional papillary thyroid cancer. Testing for this mutation may be useful for selecting initial therapy and for follow-up monitoring.
BRAF V600E mutation analysis was performed in 347 tumor samples from 314 patients with thyroid cancer (245 conventional papillary thyroid cancer, 73 follicular thyroid cancer, and 29 follicular variant of papillary thyroid cancer). The BRAF V600E mutation occurred almost exclusively in patients with conventional papillary thyroid cancer, in whom it was associated with aggressive tumor phenotype and a higher rate of recurrent and persistent disease.
The incidence of thyroid cancer has increased over the last 3 decades and more than 30,000 new cases of thyroid cancer are expected to occur in 2007.1 Differentiated thyroid cancer arises from follicular (papillary, follicular, Hürthle cell cancer) and parafollicular cells (medullary thyroid cancer). Papillary (80%) and follicular (15%) thyroid cancers account for more than 95% of all thyroid cancer cases. The increase in thyroid cancer incidence seems to be primarily due to better detection of early stage thyroid cancer because of the increasing use of sensitive imaging studies and fine needle aspiration biopsy.2 Although most of the increase in thyroid cancer incidence is due to small (<2 cm) papillary thyroid cancers (PTCs), which are commonly curable with thyroidectomy, the number of patients who die of thyroid cancer has also increased.3
Many risk classification systems have been used to estimate disease-free survival and cause-specific mortality in patients with differentiated thyroid cancer.4 These systems are mainly used to identify patients with a poor prognosis, to ensure that they receive additional treatment such as radioiodine ablation and more frequent follow-up monitoring. Unfortunately, none of these systems includes data that are completely available preoperatively to guide the extent of initial surgical resection necessary.
Moreover, most patients (80% or more) currently diagnosed with thyroid cancer have small, localized, PTC, but may receive aggressive treatment because their risk of recurrence and mortality cannot be reliably predicted preoperatively.4
On the other hand, some clinicians in Japan have suggested that patients with a solitary small (<1.0 cm) PTC may require observation only, if there is no evidence of lymph node metastasis or multifocal disease on neck ultrasound.5 Given the increasing incidence of thyroid cancer, most of which is subclinical, and the increasing number of deaths due to thyroid cancer, there is a pressing need to identify a reliable preoperative approach for stratifying patients according to risk of recurrence and death, to determine the need for and extent of surgery, as well as, the need for adjuvant therapy.
The completion of the human genome project and advances in molecular biology techniques have improved our understanding of the genetic changes that lead to carcinogenesis and have provided opportunities for identifying disease biomarkers. The recently discovered activating mutation in the gene for the B-type Raf kinase, BRAF, is the most common genetic alteration in thyroid cancer.6 BRAF is located on chromosome 7, and is the most potent activator of the mitogen-activating kinase pathway among the 3 forms of Raf kinases. BRAF-activating mutations are restricted to the kinase domain located on exons 11 and 15.7 The BRAF V600E mutation at nucleotide position 1799, previously designated as BRAF V599E, is a transversion of a thymine to adenine, which results in a conversion of valine to glutamate. The BRAF V600E mutation has been observed in 18% to 87% of thyroid cancers.6,7 It is most commonly present in PTC, and some forms of poorly differentiated thyroid cancer and anaplastic thyroid cancers that coexist with, or arise from, PTC.8
Both in vitro studies and transgenic models of BRAF suggest that the BRAF V600E mutation promotes thyroid cancer progression and is associated with invasive thyroid cancer phenotype.9,10 On the basis of these findings, several investigators have evaluated whether the presence of a BRAF V600E mutation in thyroid cancer is associated with an aggressive tumor phenotype. Some studies suggest an association between the presence of BRAF V600E mutation and poor prognostic factors, such as older age, male gender, extrathyroidal tumor invasion, lymph node and distant metastases, higher tumor stage, and even higher rates of recurrent disease.11–18 However, several investigators have not found the presence of the BRAF V600E mutation to be associated with aggressive thyroid cancer phenotype.19–23
Several reasons exist for the discrepancy as to whether there is a genotype-histologic subtype and genotype-phenotype association based on BRAF V600E mutation status in thyroid cancer. These include lack of multivariate analysis in most studies, small study cohorts, inclusion of different histologic subtypes of thyroid cancer, and lack of both follow-up data and detailed clinicopathologic analysis.
In this study, we analyzed 347 papillary and follicular thyroid cancer (FTC) tumors for the presence of BRAF V600E mutation by polymerase chain reaction (PCR) amplification and direct sequencing to determine whether there is a BRAF V600E genotype-histologic subtype and genotype-phenotype association.
METHODS
Study Cohort
Three hundred fourteen patients with thyroid cancer [245 conventional PTC, 73 FTC, and 29 follicular variant of papillary thyroid cancer (FVPTC)] and complete clinicopathologic data and follow-up were studied (Table 1). All thyroid tissue diagnoses were confirmed by permanent histologic examination and the study was approved by the Committee on Human Research at the University of California, San Francisco.
TABLE 1. Clinical and Pathologic Characteristics of Study Cohort
Thyroid Tumor Tissue and DNA Isolation
Fresh frozen (n = 251) or paraffin-embedded (n = 96) thyroid cancer tissue samples were microdissected for DNA isolation. For the paraffin-embedded tumor samples, tissue was treated with xylene at room temperature and digested with 1% sodium dodecyl sulfate and 0.5 mg/mL proteinase K at 48°C for 1 hour. For both fresh frozen tissue and paraffin-embedded tissue, genomic DNA was extracted by using standard phenol-chloroform extraction (DNA STAT-60, Tel-Test, Inc.) and ethanol precipitation. The quality and quantity of the extracted DNA were determined by spectrophotometry (NanoDrop 3.0, NanoDrop, Inc., Wilmington, DE).
BRAF PCR and Sequencing
The most common T1799A transversion mutation (BRAF V600E) was studied by direct sequencing after PCR amplification of exon 15 of the BRAF gene. Fifty nanograms of DNA was amplified using the following primers: forward −5′TGTAAAACGACGGCCAGTCA TAA TGC TTG CTC TGA TAG GA 3′; reverse −5′ AGCGGATAACAATTTCACACAGGC CAA AAA TTT AAT CAG TGG A 3′. The PCR products were run on a 1.5% agarose gel to determine the quality of the PCR product. The PCR products before sequencing were treated with Exonuclease (USB Corp., Cleveland, OH) to eliminate excess primers and Shrimp Alkaline Phosphatase (USB Corp.) to dephosphorylate excess deoxynucleotides. Sequencing was performed with a colorimetric method using the BigDye Terminator v3.1 Cycle Sequencing Kit on an ABI PRISM 3730 genetic analyzer (AppliedBiosystems, Foster City, CA).
Data Analysis
χ2 and Fisher exact test tests were used for categorical data, and Kruskal-Wallis and Mann-Whitney tests were used for nonparametric data. Data are reported as mean ± SD or number (percent). Variables significant on univariate analysis were included in a multivariate logistic regression analysis. The specific variables studied were age, sex, histologic subtype of thyroid cancer, tumor size, extent of tumor differentiation and multicentricity, lymph node and distant metastases, TNM stage, the Age, Metastases, Extrathyroidal invasion, and Size (AMES) risk group, surgical treatment, and I-131 treatment. Data were analyzed with Stat View (Cary, NC) statistical software. The observed differences were assumed to be statistically significant if the probability of chance occurrence was less than 0.05.
RESULTS
Prevalence of BRAF V600E Mutation in Thyroid Cancer
The BRAF V600E mutation was detected in 51% (126 of 245) of conventional PTCs, 24% (7 of 29) of FVPTCs, and 1% (1 of 73) FTCs (P ≤ 0.005 between all 3 histologic groups).
Multiple tumor samples (primary vs. one or more lymph node metastases) from 36 patients with conventional PTC were also analyzed for BRAF V600E mutation. In 32 patients with a primary tumor and 1 lymph node metastasis: 13 had BRAF V600E mutation in both samples, 14 had no BRAF V600E mutation in both tumor samples, and 5 had discordant BRAF V600E mutation status (3 positive in primary tumor and 2 positive in the lymph nodes). Three patients had their primary tumor and 2 lymph node metastases analyzed: 2 of 3 had BRAF V600E mutation in all the samples, and 1 patient had no BRAF V600E mutation in all the samples. The 1 patient with a primary tumor and 3 lymph node metastases had the BRAF V600E mutation in all the samples.
BRAF V600E Mutation Status and Thyroid Cancer Phenotype by Histologic Subtype
Comparison of BRAF V600E mutation status with clinical and pathologic factors in patients with PTC and the FVPTC revealed significant associations of BRAF V600E with older age (P = 0.0288), AMES risk group (P = 0.0448), and recurrent and persistent disease (P = 0.0326) with a trend towards a higher rate of lymph node metastasis (P = 0.0660) and higher TNM stage (I and II vs. III and IV, P = 0.0703) (Table 2). In patients with conventional PTC, BRAF V600E mutation was associated with older age (P = 0.0381), lymph node metastasis (P = 0.0323), distant metastasis (P = 0.045), higher TNM stage (I and II vs. III and IV, P = 0.0389), and recurrent and persistent disease (P = 0.009) (Table 2).
TABLE 2. Associations Between the BRAF V600E Mutation and Clinicopathologic Characteristics of Papillary Thyroid Cancer
BRAF V600E Mutation Status as a Prognostic Factor in Conventional PTC
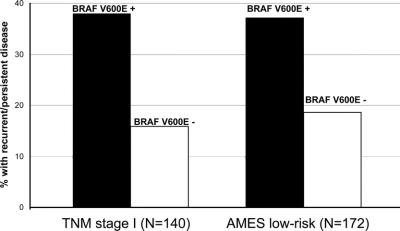
According to our univariate analysis, BRAF V600E mutation, gender, tumor size, lymph node metastasis, extrathyroidal invasion, TNM stage, AMES risk group, and distant metastasis were significantly associated with a higher rate of recurrent and persistent disease (Table 3). Multivariate analysis showed that BRAF V600E mutation [OR (95% CI) = 4.2 (1.2–14.6)] and lymph node metastasis [OR (95% CI) = 7.75 (2.1–28.5)] were independently associated with recurrent and persistent disease (Table 3). The presence of the BRAF V600E mutation was also associated with a higher rate of recurrent and persistent disease, even when we only considered TNM stage I (P = 0.0033) and AMES low-risk conventional PTC (P = 0.0065) (Fig. 1). Extent of thyroidectomy and use of postoperative radioiodine therapy did not differ significantly between patients whose tumors had the BRAF V600E mutation and those whose tumors did not. Length of follow-up time did not differ significantly by BRAF V600E mutation status or by recurrent and persistent disease status.
TABLE 3. Risk Factors for Recurrent and Persistent Conventional Papillary Thyroid Cancer
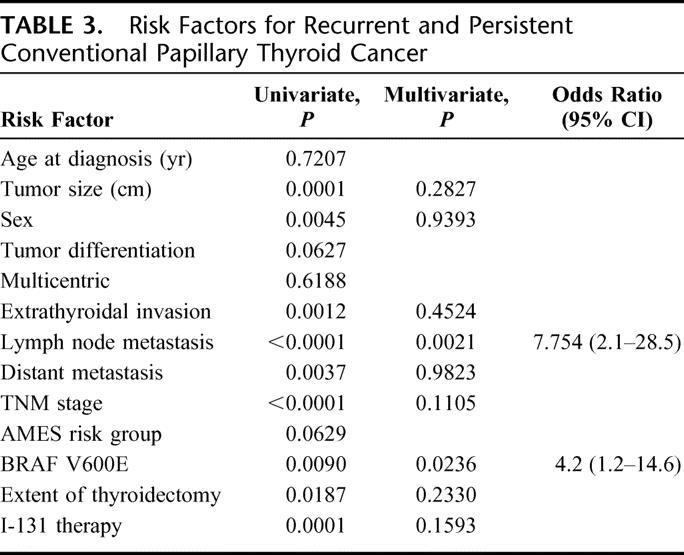
FIGURE 1. BRAF V600E mutation and recurrent and persistent conventional papillary thyroid cancer by TNM stage and AMES risk group.
DISCUSSION
This study shows that the BRAF V600E mutation was significantly more common in conventional PTC than in the FVPTC or FTC. It also indicates that the presence of the BRAF V600E mutation in both conventional PTC and the follicular variant was associated with older age, high-risk AMES tumor, and higher rates of recurrent and persistent disease. Most importantly, the presence of the mutation in conventional PTC was associated with older age and lymph node and distant metastases, and was an independent prognostic factor for recurrent and persistent disease.
Although most studies report that BRAF V600E mutation is common in PTC, reported frequencies vary from 18% to 87%.7,11 Although some investigators have suggested that technical and geographic factors may account for the differences in reported prevalence of BRAF V600E mutation, the more likely reason is a lack of histologic subtype classifications. In our study, the BRAF V600E mutation was present in 51% of conventional PTCs and in 24% of the follicular variant, findings that are similar to those of other studies, when stratified by histologic subtype.6,12,15,16,19,20 Although no BRAF V600E mutation has been previously observed in FTCs or in benign thyroid tumors, we found 1 FTC that had a BRAF V600E mutation. Because of this, the tumor sample in question was rereviewed, confirming it was a FTC. Interestingly, some of the tumor cells showed intranuclear pseudoinclusions and nuclear grooves raising the possibility of FVPTC, but the growth pattern with clear capsular invasion and vascular invasion was most characteristic of FTC.
Some investigators have reported a higher rate of BRAF V600E mutation in PTCs from older patients.12,13,24 We also found that primary tumors from older patients with conventional and follicular variant PTC were significantly more likely to have a BRAF V600E mutation. The youngest patient to have a BRAF V600E mutation in our cohort was 16 years old and only 4 of 25 patients who were 21 years old or younger had a BRAF V600E mutation in the primary tumor. The reason for this association is unclear, but it is tempting to speculate that because BRAF V600E mutation occurs early in papillary thyroid carcinogenesis, it is more likely to be prevalent in tumors from older patients.
In some of our patients with conventional PTC, BRAF V600E mutational status differed between the primary tumor and the lymph node metastasis. Although most of the cases did not have heterogeneity in BRAF V600E mutation, about 16% did. These findings are consistent with those of other investigators and likely reflect different clonal origins of PTC.25,26
Although the prognostic value of BRAF V600E mutation in PTC is controversial, several investigators have shown an association between the mutation and aggressive tumor phenotype, such as older age, higher prevalence in men, extrathyroidal tumor invasion, lymph node and distant metastases, higher tumor stage and poorly differentiated tumors.11–18 More recently, Xing and colleagues reported that tumors with the BRAF V600E mutation were associated with higher rates of recurrent disease and lower avidity of radioiodine.15 Among the reasons proposed for these discordant findings are study cohort size, geographic area, methodologies, lack of multivariate analysis to account for confounding factors, inclusion of nonconventional PTC, and insufficient follow-up time to determine outcome. To address many of these study design limitations, we analyzed more than 300 thyroid cancer samples, which were stratified by histologic subtype and came from patients who received similar treatment and had relatively long follow-up time (6 years). We also used multivariate analyses to account for confounding factors. When we examined conventional PTC and the follicular variant, we found an association between BRAF V600E mutation and older age, high-risk AMES tumors, and recurrent and persistent disease. Interestingly, when we considered only the conventional PTC, which is most common, BRAF V600E mutation was significantly associated with older age, lymph node and distant metastases, higher TNM stage, and a higher rate of recurrent and persistent disease. Most importantly, the presence of lymph node metastasis and BRAF V600E mutation were independently associated with a higher rate of recurrent and persistent disease in our cohort. Our findings therefore suggest that the BRAF V600E mutation is indeed associated with aggressive phenotype and may be an independent marker for recurrent or persistent disease for conventional PTC. Therefore, preoperative testing for BRAF V600E mutation in thyroid nodule fine needle aspiration biopsy may be useful for predicting disease aggressiveness when determining the need for and extent of surgery, as well as, the need for adjuvant therapy.
In summary, BRAF V600E mutation is most commonly present in conventional PTC and is associated with aggressive tumor phenotype and higher risk of recurrent and persistent disease in patients with conventional PTC. Testing for this mutation may be useful for selecting initial therapy and for follow-up monitoring.
Discussions
Dr. Richard A. Prinz (Chicago, Illinois): I would like to compliment Dr. Kebebew and his coworkers on their studies of the molecular biology of thyroid cancer. They have shown that 51% of pure papillary thyroid cancers were BRAF positive, which is similar to the 38% we have reported in our own patient population with papillary thyroid cancer.
The authors note that this rate has varied widely. In various series ranged from 18% to 87%, with lower rates generally found in reports from Asian countries. So I would like to ask if they have any explanation for this lower rate of BRAF positive tumors in these Asian series. Is this a technical or a patient selection issue, or is there a biologic difference in these tumors in different parts of the world?
You have evaluated both paraffin-embedded and fresh frozen tumor samples. Was there any difference in the quality or quantity of BRAF mutation according to the sample source? Did you study any tumors that had both paraffin-embedded and fresh frozen samples and did the results coincide?
As you mentioned, you had 5 discordant samples in which either the primary or metastatic lesion was BRAF positive but not both. Can you speculate on why the primary was positive but not the metastases, since you are postulating BRAF positivity indicates a more aggressive phenotype?
In our study, we found that 37% of our BRAF positive tumors also had a mutation of RET PTC, while none of our BRAF positive tumors had mutations in the 3 forms of RAS. Have you looked at any overlap with other gene mutations?
Finally, the extent of thyroidectomy and the use of radioactive iodine did not differ significantly between BRAF positive and BRAF negative tumors in your study. Do you think BRAF positivity or negativity should influence our decision making in terms of the extent of operation including the performance of total thyroidectomy and of Level VI node dissection and in terms of the use of radioiodine therapy?
Also, our endocrinologists use radioiodine treatment on almost all patients. Will this make any difference at all? Is there any clinical relevance in your findings when it comes to treating patients with papillary thyroid cancer?
Dr. Electron Kebebew (San Francisco, California): Why the difference in prevalence of BRAF mutation amongst the different studies? I think it is difficult to know for sure. Certainly technical factors could account for it. But I think the most important factor is that few studies have stratified the prevalence of BRAF mutation by the different histologic subtypes of thyroid cancer.
In your second question you asked whether there was a difference between the paraffin and frozen tissues. Certainly, the quality of the DNA was much better in the frozen tissue. We did not see a difference in the rates of BRAF mutation positive results between the different sources of samples.
The discordance in BRAF mutation in primary tumor versus lymph node metastasis is an interesting issue and there have been several studies that evaluated multicentric papillary thyroid cancer and demonstrate that about one-third of these cases were heterogeneous for BRAF mutation status, meaning 1 area positive, 1 area negative. I think, as suggested by these investigators, this may represent different clonal origins of papillary thyroid cancer.
As far as the overlap in looking at other common genetic changes, we have only looked at RAS (H, K, N) mutations in these same samples, and did not have any cases that were BRAF positive, and positive for the 3 different RAS mutations. We have not looked at RET/PTC, and I think that would be interesting to look at but others have suggested them as mutually exclusive in most cases.
As far as the clinical relevance of testing BRAF mutation status in clinical samples, I think it could be a useful adjunct to current clinical risk stratification systems when making surgical or medical management decisions. This will be even more of a pressing issue with the increasing incidence of thyroid cancer, mostly due to the diagnosis of small, localized papillary thyroid cancer. As you mentioned some surgeons suggest that a prophylactic central neck node dissection be performed at the initial operation because of the use of postoperative ultrasound and thyroglobulin level for monitoring are detecting disease that would not be detected in the past. And I think it is precisely for this situation that it would be very useful in at least justifying a prophylactic central lymph node dissection in those cases that are more likely to have persistent/recurrent disease based on BRAF mutation status if the tumor is only localized to the thyroid gland without lymph node disease.
BRAF testing could also be useful for deciding for which patient with low-risk disease you are more likely to use low- versus high-dose radioiodine ablation therapy, or which patients that have detectable postoperative thyroglobulin levels you need to monitor aggressively for recurrence.
Footnotes
Supported by the Robert Wood Johnson Foundation, American Cancer Society Research Scholars Grant, Hellman Family Grant, the University of California Cancer Research Committee, and the National Institutes of Health (NIH 1R21 CA 118688-01; to E.K.).
Reprints: Electron Kebebew, MD, FACS, University of California, San Francisco, Department of Surgery, Box 1674, UCSF/Mount Zion Medical Center, San Francisco, CA 94143-1674. E-mail: kebebewe@surgery.ucsf.edu.
REFERENCES
- 1.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Jama. 2006;295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG HD, Kraphco M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Eisner MP, Hornoer MJ, Howlader N, Hayat M, Hankey BF. SEER Cancer Statistics Review, 1975–2003. Bethesda, MD: National Cancer Institute; November 2005. Available at http://seer.cancer.gov/csr/1975.
- 4.Kebebew E, Clark OH. Differentiated thyroid cancer: “complete” rational approach. World J Surg. 2000;24:942–951. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–387. [DOI] [PubMed] [Google Scholar]
- 6.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. [DOI] [PubMed] [Google Scholar]
- 7.Trovisco V, Soares P, Sobrinho-Simoes M. B-RAF mutations in the etiopathogenesis, diagnosis, and prognosis of thyroid carcinomas. Hum Pathol. 2006;37:781–786. [DOI] [PubMed] [Google Scholar]
- 8.Begum S, Rosenbaum E, Henrique R, Cohen Y, Sidransky D, Westra WH. BRAF mutations in anaplastic thyroid carcinoma: implications for tumor origin, diagnosis and treatment. Mod Pathol. 2004;17:1359–1363. [DOI] [PubMed] [Google Scholar]
- 9.Knauf JA, Ma X, Smith EP, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;15(65):4238–4245. [DOI] [PubMed] [Google Scholar]
- 10.Mesa C Jr, Mirza M, Mitsutake N, et al. Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer Res. 2006;1(66):6521–6529. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. 2003;1(63):4561–4567. [PubMed] [Google Scholar]
- 12.Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. [DOI] [PubMed] [Google Scholar]
- 13.Trovisco V, Soares P, Preto A, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch. 2005;446:589–595. [DOI] [PubMed] [Google Scholar]
- 14.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2006;65:364–368. [DOI] [PubMed] [Google Scholar]
- 15.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Giuliano AE, Turner RR, et al. Lymphatic mapping establishes the role of BRAF gene mutation in papillary thyroid carcinoma. Ann Surg. 2006;244:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo YS, Li S, Song JH, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. 2006;91:3667–3670. [DOI] [PubMed] [Google Scholar]
- 18.Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. [DOI] [PubMed] [Google Scholar]
- 19.Fugazzola L, Mannavola D, Cirello V, et al. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf). 2004;61:239–243. [DOI] [PubMed] [Google Scholar]
- 20.Kim TY, Kim WB, Song JY, et al. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2005;63:588–593. [DOI] [PubMed] [Google Scholar]
- 21.Liu RT, Chen YJ, Chou FF, et al. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin Endocrinol (Oxf). Oct. 2005;63:461–466. [DOI] [PubMed]
- 22.Puxeddu E, Moretti S, Elisei R, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–2420. [DOI] [PubMed] [Google Scholar]
- 23.Sedliarou I, Saenko V, Lantsov D, et al. The BRAFT1796A transversion is a prevalent mutational event in human thyroid microcarcinoma. Int J Oncol. 2004;25:1729–1735. [PubMed] [Google Scholar]
- 24.Fugazzola L, Puxeddu E, Avenia N, et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr Relat Cancer. 2006;13:455–464. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Park YJ, Lee YJ, et al. Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer. 2006;107:1831–1838. [DOI] [PubMed] [Google Scholar]
- 26.Shattuck TM, Westra WH, Ladenson PW, Arnold A. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. N Engl J Med. 2005;352:2406–2412. [DOI] [PubMed] [Google Scholar]