Abstract
Hepatitis C virus (HCV), a single-stranded positive-sense RNA virus of the Flaviviridae family, infects more than 170 million people worldwide and is the leading cause of liver failure in the United States. A unique feature of HCV is that the viral life cycle depends on cholesterol metabolism in host cells. This review summarizes the cholesterol metabolic pathways that are required for the replication, secretion, and entry of HCV. The potential application of drugs that alter host cholesterol metabolism in treating HCV infection is also discussed.
Introduction
Hepatitis C virus (HCV) exacts a heavy toll on public health. Approximately 3% of the world population is infected persistently with HCV [1]. According to the Centers for Disease Control and Prevention, 4.1 million Americans (1.6% of the United States population) are estimated to be infected by HCV, 3.2 million of whom become chronically infected [2]. These individuals account for most of the cases of liver failure in the United States [3]. Owing to its genomic variation, HCV variants are divided into six genotypes (genotypes 1–6), which differ from each other by 31% to 33% at the nucleotide level [4]. Genotype 1 HCV is the most prevalent viral variant found in the United States. This genotype is also less sensitive to the current interferon-based therapies, with only about 50% of patients responding to such treatment [5]. Thus, new therapeutic strategies to combat HCV infection are needed.
HCV is a single-stranded positive-sense RNA virus of the Flaviviridae family [6]. The 9.6-kilobase HCV genome encodes a single polyprotein that is post-translationally processed into at least ten structural and nonstructural (NS) proteins [7]. The amino-terminal third of the polyprotein encodes the virion structural proteins: core, E1, and E2, which are followed by a small integral membrane protein p7 that functions as an ion channel [8]. The remainder of the genome encodes the nonstructural (NS) proteins NS2, NS3, NS4A, NS4B, NS5A, and NS5B. Except for NS2, all of the other NS proteins are required for efficient viral RNA replication. These NS proteins (NS3–NS5B) form a viral replication complex on intracellular membrane vesicles, leading to a structure termed the membranous web [9,10].
For reasons still unknown, most clinically isolated HCV is difficult to replicate in cultured cells [7]. Thus, cells harboring HCV subgenomic replicons are widely used to study HCV replication. These replicons consist of HCV RNA engineered to express a selectable marker gene, neo, in place of the structural protein-coding region. A heterologous viral internal ribosomal entry site is inserted after the neomycin resistance cassette to direct translation of viral NS proteins (NS3–NS5B). When human hepatoma Huh7 cells were transfected with HCV replicon RNA and selected with G418, a cell line was established in which HCV RNA was constantly replicated [11]. This system, although very effective in studying HCV replication, is not able to produce infectious HCV particles. Recently, a strain of genotype 2 HCV was shown to be capable of replicating in Huh7 cells and producing HCV particles that are infectious to cultured cells [12–14]. The HCV particles produced from cell culture (referred to as HCVcc) were able to establish long-term infections in chimpanzees and in mice containing human liver grafts [15]. Moreover, virus recovered from these animals was still infectious in cell culture [15]. For reasons still unknown, only this strain of HCV or its derivative can be cultured in Huh7 cells to generate infectious viral particles.
HCV infection is mainly restricted to hepatocytes [1], which play a vital role in mammalian cholesterol homeostasis. Like all mammalian cells, hepatocytes are able to acquire cholesterol through two pathways. Cholesterol can be synthesized from acetyl-CoA via the mevalonate pathway [16], which also generates several isoprenoids, including farnesyl and geranylgeranyl lipids that are covalently attached to the COOH-terminus of certain proteins [17] (Figure 1). Cells can also acquire low density lipoprotein (LDL)–associated cholesterol in serum through LDL receptor–mediated endocytosis [18]. The LDL receptor binds to LDL particles, which are internalized via the clathrin-dependent pathway of receptor-mediated endocytosis. Following endocytosis, the lipoprotein is degraded in lysosomes and cholesterol in LDL is released into the cells [18].
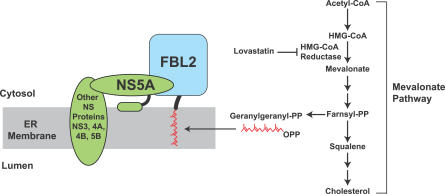
Figure 1. Requirement of the Mevalonate Pathway for HCV Replication.
Cells are capable of synthesizing cholesterol through the mevalonate pathway. This pathway also produces geranylgeranyl lipid, which is attached to the COOH-terminus of FBL2. Geranylgeranylated FBL2 binds to NS5A, an interaction required for HCV RNA replication. Lovastatin, an inhibitor of HMG CoA reductase, blocks the entire mevalonate pathway. As a result, cells are depleted of geranylgeranyl lipid and replication of HCV is inhibited. PP, pyrophosphate.
In mice, hepatocytes can also acquire a substantial amount of cholesterol through scavenger receptor class B type I (SR-BI)–mediated uptake of cholesterol from high density lipoprotein (HDL) particles [19]. In humans, hepatic cholesterol contributed by HDL is not as significant. This is because humans, but not mice, express cholesteryl ester transfer protein, which transfers cholesterol from HDL to LDL or its precursor, very low density lipoprotein (VLDL) [20]. As a result, the amount of HDL-associated cholesterol is much lower in humans compared to mice, in which HDL particles transport most of the plasma cholesterol.
Hepatocytes play a crucial role in regulating mammalian cholesterol metabolism by exporting cholesterol together with triglycerides through secretion of VLDL (Figure 2). The assembly of VLDL begins with synthesis of full length apolipoprotein B (apoB), a 540-kDa protein that confers structural integrity to VLDL [21]. Nascent apoB is then fused with lipid droplets that are rich in triglyceride and cholesteryl esters in the lumen of the endoplasmic reticulum (ER). While most cells contain cytosolic lipid droplets, hepatocytes, as well as other cells producing lipoprotein particles that contain apoB, have lipid droplets in the lumen of the ER [22]. Fusion between lipid droplets and apoB requires the activity of microsomal triglyceride transfer protein (MTP) [23]. Without MTP-mediated lipid transfer, the secretion of apoB is blocked and the protein is degraded in cells [24]. The importance of MTP in VLDL assembly is demonstrated by the observations that genetic defects in MTP severely reduce VLDL secretion [25,26]. The resulting VLDL particles are composed of a hydrophobic core of triglycerides and cholesteryl esters surrounded by a surface coat containing phospholipids, free cholesterol, and two predominant lipoproteins, apoB and apolipoprotein E (apoE). VLDL is secreted out of cells through exocytosis [27].
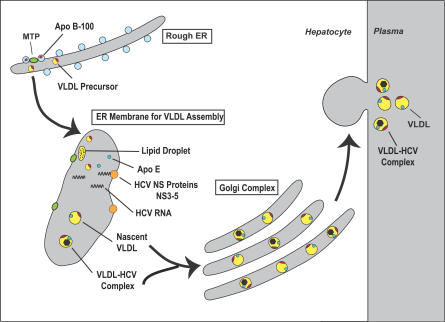
Figure 2. HCV Replicates on ER Membranes Involved in the Assembly of VLDL and Is Secreted Together with VLDL.
The assembly of VLDL begins with the synthesis of apoB in the rough ER, resulting in the formation of a VLDL precursor that contains only a small amount of lipid. In the lumen of the ER, this precursor is fused with lipid droplets (enriched in triglyceride and cholesterol) to generate nascent VLDL. This reaction is mediated by MTP. The nascent VLDL particles, which contain both apoB and apoE, are secreted into plasma through exocytosis. The ER membranes involved in the assembly of VLDL are also enriched in HCV NS proteins and RNA. Replication of HCV on these membranes might allow the virus to attach to or become incorporated into VLDL so that HCV is secreted together with VLDL.
Nascent VLDL particles released into plasma are not ligands for the LDL receptor [28]. These lipoprotein particles are substrates for lipoprotein lipase, which hydrolyzes the triglycerides in the core of the lipoprotein particles [29]. A large proportion (∼70%) of the resulting particles, called intermediate density lipoprotein (IDL) particles, is efficiently removed from plasma by the LDL receptor on hepatocytes [18]. The remaining IDL particles in the circulation are converted to LDL particles by a reaction catalyzed by hepatic lipase, which further reduces the amount of triglycerides in the lipoprotein particles [30]. Once formed, LDL is taken up by the LDL receptor on hepatic as well as nonhepatic cells [18].
Requirement of the Mevalonate Pathway in HCV RNA Replication
The first clue that the mevalonate pathway is involved in HCV RNA replication was the finding that treatment of cultured cells with lovastatin, a cholesterol-lowering drug that inhibits 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase, the rate-limiting enzyme in the entire mevalonate pathway [16], inhibited HCV RNA replication [31]. Similar results were subsequently obtained by others [32–34].
The product of the mevalonate pathway required for HCV RNA replication turns out to be a geranylgeranyl lipid, as shown by experiments in which the inhibition of HCV RNA replication by lovastatin was overcome by the addition of geranylgeraniol, but not farnesol or cholesterol [31,32]. Geranylgeranyl serves as a lipid substrate for protein geranylgeranylation, a post-translational modification that covalently attaches geranylgeranyl to various cellular proteins to facilitate their membrane association [17]. Thus, it appears that one or more geranylgeranylated proteins are required for HCV RNA replication. This notion is further supported by the observation that HCV replication could be blocked by an inhibitor of geranylgeranyl transferase I [31], an enzyme that transfers geranylgeranyl groups to cellular proteins [17].
Inasmuch as HCV does not encode a viral protein that contains the signature COOH-terminal CAAX motif (i.e., cysteine-X-X-leucine or isoleucine, in which X is an aliphatic amino acid) that specifies geranylgeranylation, it was hypothesized that a geranylgeranylated host protein may be required for HCV RNA replication. In this regard, a 50-kDa geranylgeranylated host protein was found to co-immunoprecipitate with the viral protein NS5A [35]. With the knowledge of the molecular weight of the protein and the sequence motif that specifies geranylgeranylation, this protein was identified as FBL2 by a bioinformatics approach [35]. Geranylgeranylation of FBL2 appears to be critical for HCV replication because the association between FBL2 and NS5A depends on geranylgeranylation of FBL2, and this interaction is required for HCV RNA replication [35] (Figure 1). The exact role played by FBL2 in HCV RNA replication remains unclear because the physiological function of FBL2 is not known. FBL2 contains an F-box, a domain known to be involved in protein ubiquitination [36]. Thus, it is possible that protein ubiquitination may play a role in HCV RNA replication. It should be noted that all of the aforementioned studies used genotype 1 HCV replicons. It is not known whether geranylgeranylation of host proteins is required for replication of other genotypes of HCV.
Unlike geranylgeranyl, the requirement of cholesterol for HCV RNA replication is still under debate. The observation that addition of cholesterol failed to rescue HCV RNA replication in the presence of lovastatin suggests that cholesterol is not directly involved in HCV RNA replication [31]. However, differing results were obtained when cells were depleted of cholesterol by treatment with methyl-β-cyclodextrin, which directly extracts cholesterol from cell membranes [37]. Under these conditions, HCV RNA replication is either not affected [38] or reduced by 50% [39]. The inconsistency in these results could arise from a secondary effect of general cellular toxicity that occurs when cells are depleted of cholesterol, or from a difference in the HCV genotype used in these studies.
Requirement for VLDL Assembly in HCV Secretion
Like all positive-strand RNA viruses, HCV RNA replication occurs in association with cytoplasmic membrane vesicles. In the case of HCV, these structures (membranous webs) have been visualized in cultured human hepatoma Huh7 cells that harbor a subgenomic replicon of HCV [9,40]. Recently, membrane vesicles containing the HCV replication complex from Huh7 cells that harbor an HCV replicon were isolated [41]. Proteomic analysis revealed that these vesicles are enriched in apoB, apoE, and MTP, proteins known to be required for the assembly of VLDL [41]. Interestingly, VLDL synthesis is not required for HCV RNA replication [41]. This result is consistent with the previous findings that HCV RNA can replicate in HeLa and HEK-293 cells [42–44], which do not produce VLDL. The reason for co-localization of the HCV replication and VLDL assembly appears to lie in a requirement for co-assembly or secretion of VLDL and HCV particles (Figure 2). Thus, When Huh7 cells that constitutively produce infectious HCV [45] were treated with an MTP inhibitor or siRNA targeting apoB, the secretion of VLDL and HCV were both inhibited [41].
Although VLDL secretion is required for HCV production in cultured cells, this requirement has not been demonstrated in HCV-infected patients. However, it was shown that HCV particles circulating in serum are in complex with VLDL [46–48]. Thus, it appears that HCV particles are attached to or incorporated into VLDL during the assembly of the lipoprotein particles and secreted together with VLDL. Although the nature of the association between HCV and VLDL remains unclear, studies with HCV isolated from infected patients suggests that HCV may reside in the lipid-rich core of VLDL, since delipidation of lipoprotein particles that contain apoB is required to observe the capsid-like structures of HCV [46,47]. If HCV indeed hides in the core of VLDL as suggested [49], it makes the virus unique in that the entire virion is not exposed to serum during circulation. If this model is correct, it will also predict that HCV has to escape from VLDL-derived lipoprotein particles during viral entry so that the structural protein E2 can interact with its cellular receptors, a step required for HCV RNA to enter cytosol (see below). Exactly what triggers the release of HCV from the lipoprotein particles is not known. If LDL receptor–mediated endocytosis is required for HCV entry (see below), the virus is likely to escape VLDL-derived particles in endocytic vesicles after the lipoprotein particles are disassembled during endocytosis. This scenario does not necessarily contradict the observation that entry of HCVcc was inhibited by antibodies targeting viral structural protein E2 [13], since these antibodies might also be included in endocytic vesicles that contain HCV. It was reported previously that immunoglobin G is able to enter clathrin-coated pits nonspecifically through fluid-phase endocytosis [50,51]. Thus, these antibodies may block HCV entry by binding to the viral structural protein after the virus is released from the lipoprotein particles in endocytic vesicles.
The observation that HCV replicates on membrane vesicles involved in the assembly of VLDL might also help to explain the effect of HCV infection on hepatic release of VLDL. Infection by HCV, particularly the genotype 3 variant, leads to a reduced rate of VLDL secretion [52,53]. The reduction in VLDL secretion could arise from inhibition of MTP by viral proteins. It has been reported that mRNA levels of MTP are reduced in Huh7 cells harboring HCV replicons [54], and that transgenic mice expressing HCV core protein in livers have reduced MTP activity [55]. However, the reduction in MTP activity has not been demonstrated in cells or patients that are infected by HCV. The role of reduced VLDL secretion in HCV infection is currently unknown. It is possible that by delaying the secretion of VLDL, HCV might have enough time to replicate and assemble into VLDL.
Besides liver, intestine is the only other tissue that produces apoB-containing lipoprotein particles. These particles, called chylomicrons, contain a shorter form of apoB [56]. Although HCV infection in intestinal cells was reported in a fraction of HCV-infected patients [57,58], the amount of HCV detected in intestine was much lower than that in liver [58]. However, in patients that had HCV infection in intestine, HCV particles circulating in serum were in complex with chylomicrons [59]. Thus, it appears that intestinal cells are able to export HCV, but they might not contain cellular factors that support efficient entry and/or replication of HCV.
Requirement of Lipoprotein Receptors for HCV Entry
HCV enters cells through multiple cellular receptors. CD81, which directly binds to HCV structural protein E2 [60,61], is required for HCV to enter host cells [13,62]. Interestingly, the amount of CD81 expressed on the cell surface is affected by cellular cholesterol content. When cells were depleted of cholesterol by treatment with methyl-β-cyclodextrin, the amount of CD81 located on plasma membrane was reduced, resulting in a reduction in HCV entry [38]. Recently, claudin-1 was identified as a receptor functioning at a step later than CD81 [63]. Both CD81 and claudin-1 have been demonstrated to be required for HCV entry by experiments showing that viral entry was blocked in cells that do not express either one of these proteins, and that reintroduction of the protein into the cells specifically restores the viral entry [13,63]. These receptors appear to act at later steps of HCV entry, because CD81 is not required for HCV to bind to the cell surface [64] and is not known to initiate the clathrin-mediated endocytosis that is required for entry of HCV [65]. Considering the association between HCV and VLDL-derived lipoprotein particles, lipoprotein receptors might function at earlier steps in HCV entry.
The LDL receptor, which plays a predominant role in acquiring VLDL-derived lipoprotein particles through clathrin-mediated endocytosis in hepatic cells [66,67], has been reported to be involved in HCV entry. These studies demonstrated that cellular binding or uptake of HCV particles isolated from infected patients correlated with LDL receptor activity on the cell surface [47,68–70]. In contrast to the results with genuine HCV isolated from serum of HCV-infected patients, HCV pseudo particles (HCVpp), which were assembled by displaying HCV structural proteins E1and E2 onto retroviral core particles, did not require the LDL receptor for their entry [71]. These results do not argue against an LDL receptor-mediated uptake of HCV since HCVpp were produced in HEK-293 cells that do not produce VLDL [71]. These results also suggest that virus-associated lipoproteins are required for LDL receptor–mediated binding of HCV.
Nevertheless, the role of the LDL receptor in HCV entry is still uncertain, because cellular binding or uptake of HCV in all of the aforementioned studies did not result in viral infection. This outcome could be explained by the difficulty of most clinically isolated HCV to replicate in cultured cells [7]. However, the possibility that LDL receptor–mediated viral entry does not lead to viral infection cannot be ruled out. More studies using HCVcc that effectively infect cultured cells are needed to demonstrate the requirement of the LDL receptor in HCV entry.
Mutations disrupting the function of the LDL receptor produce autosomal dominant familial hypercholesterolemia, which affects 0.2% of the world population [72]. Affected individuals have elevated plasma levels of LDL cholesterol, which causes premature coronary atherosclerosis [72]. Mutations in LDL receptors might protect affected individuals from HCV infection, if the LDL receptor is indeed involved in HCV entry. An analysis comparing the frequency of HCV infection in people expressing normal LDL receptor versus those affected by familial hypercholesterolemia will be needed to address the question.
Another lipoprotein receptor that is implicated in HCV entry is SR-BI, which binds to HDL [73] and oxidized LDL [74,75]. The involvement of SR-BI in HCV entry is indicated by the observations that entry of HCVcc to cells was blocked by antibodies directed against SR-BI [38,76] and inhibited by excessive amounts of oxidized LDL [77]. However, it is not known whether HCV entry is blocked in cells that do not express SR-BI. Unlike with the LDL receptor, the interaction between HCV and SR-BI may not depend on lipoproteins associated with the virus because the HCV structural protein E2 directly binds to SR-BI [78]. This notion is further supported by observations that entry of HCVpp, which does not associate with VLDL, required SR-BI [79–82].
Potential Application of Drugs That Target Cholesterol Metabolic Pathways in Treating HCV Infection
A major obstacle in combating HCV infection is that the fidelity of the viral replication machinery is notoriously low, thus enabling the virus to quickly develop mutations that resist compounds targeting viral enzymes [83]. Therefore, drugs targeting the host proteins required for HCV infection may be more effective in combating the viral infection. A unique aspect of HCV that has not been observed in other viruses is that the entire viral life cycle is associated with cholesterol metabolism in host cells. Thus, drugs that target cholesterol metabolism might be useful in treating HCV infection.
Treatment of cells with statins, the widely used cholesterol-lowering drugs, has been reported to inhibit HCV RNA replication [31–34] by depletion of geranylgeranyl lipids [31,32]. However, the doses of statins currently used to treat hypercholesterolemia by inhibiting cholesterol synthesis, thereby stimulating expression of the LDL receptor in the liver, are not high enough to inhibit the synthesis of geranylgeranyl lipid. Applying statins to treat HCV will require much higher doses and would likely cause toxicity in the liver and other organs.
Another class of drugs designed for treating hypercholesterolemia blocks the assembly and secretion of VLDL. These drugs may also be effective in treating HCV infection because they inhibit production of HCV particles from infected cells [41]. In this regard, antisense RNA drugs targeting apoB [84] and several MTP inhibitors [85,86] have already been tested in clinical trails because of their ability to block VLDL secretion, thereby lowering the plasma levels of VLDL triglycerides and LDL cholesterol. Long-term treatment with MTP inhibitors led to the toxic accumulation of fat in livers [85,86], thus hampering the approval of these drugs for the treatment hypercholesterolemia on a long-term basis. However, short-term treatment (up to several weeks) reduced the plasma level of VLDL with only minor adverse effects, which disappeared after drug removal [85]. It will be interesting to examine whether short-term treatment with MTP inhibitors is beneficial in treating HCV infection.
Supporting Information
Accession Numbers
The National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) accession numbers for human apoB, apoE, CD81, claudin-1, FBL2, LDL receptor, MTP, and SR-BI are NP_000375, NP_000032, NP_004347, NP_066924, NP_036289, NP_000518, NP_000244, and NP_005496, respectively.
Acknowledgments
I thank Helen Hobbs, Jay Horton, and Joseph Goldstein for their critical comments to the manuscript.
Abbreviations
- apo
apolipoprotein
- ER
endoplasmic reticulum
- HCV
hepatitis C virus
- HDL
high density lipoprotein
- HMG CoA
3-hydroxy-3-methylglutaryl coenzyme A
- IDL
intermediate density lipoprotein
- LDL
low density lipoprotein
- MTP
microsomal triglyceride transfer protein
- NS
nonstructural
- SR-BI
scavenger receptor class B type I
- VLDL
very low density lipoprotein
Footnotes
Jin Ye is with the Department of Molecular Genetics, University of Texas Southwestern Medical Center. Dallas, Texas, United States of America. Email: jin.ye@utsouthwestern.edu
Author contributions. JY wrote the article.
Funding. JY is supported by research grants from the National Institutes of Health (HL-20948).
Competing interests. The author has declared that no competing interests exist.
References
- Chisari FV. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436:930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Viral hepatitis C fact sheet. 2006. Available: http://www.cdc.gov/ncidod/diseases/hepatitis/c/fact.htm. Accessed 5 August 2007.
- Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973–978. doi: 10.1038/nature04083. [DOI] [PubMed] [Google Scholar]
- Zein NN. Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev. 2000;13:223–235. doi: 10.1128/cmr.13.2.223-235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- Appel N, Schaller T, Penin F, Bartenschlager R. From structure to function: New insights into hepatitis C virus RNA replication. J Biol Chem. 2006;281:9833–9836. doi: 10.1074/jbc.R500026200. [DOI] [PubMed] [Google Scholar]
- Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- Pavlovic D, Neville DCA, Argaud O, Blumberg B, Dwek RA, et al. The hepatitis C virus p7 protein forms an ion channel that is inhibited by long-alkyl-chain iminosugar derivatives. Proc Natl Acad Sci U S A. 2003;100:6104–6108. doi: 10.1073/pnas.1031527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R, Egger D, Lohmann V, Bartenschlager R, Blum HE, et al. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J Virol. 2003;77:5487–5492. doi: 10.1128/JVI.77.9.5487-5492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger D, Wolk B, Gosert R, Bianchi L, Blum HE, et al. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann V, Körner F, Koch JO, Herian U, Theilmann L, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci U S A. 2006;103:3805–3809. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RGW, Russell DW, Schneider WJ. Receptor-mediated endocytosis: Concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Krieger M. Charting the fate of the “good cholesterol”: Identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- de Grooth GJ, Klerkx AHEM, Stroes ESG, Stalenhoef AFH, Kastelein JJP, et al. A review of CETP and its relation to atherosclerosis. J Lipid Res. 2004;45:1967–1974. doi: 10.1194/jlr.R400007-JLR200. [DOI] [PubMed] [Google Scholar]
- Olofsson SO, Boren J. Apolipoprotein B: A clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J Intern Med. 2005;258:395–410. doi: 10.1111/j.1365-2796.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Vance J. Mechanisms of lipid-body formation. TiBS. 1999;24:109–115. doi: 10.1016/s0968-0004(98)01349-8. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- Avramoglu RK, Adeli K. Hepatic regulation of apolipoprotein B. Rev Endocr Metab Disord. 2004;5:293–301. doi: 10.1023/B:REMD.0000045100.66675.92. [DOI] [PubMed] [Google Scholar]
- Sharp D, Blinderman L, Combs KA, Kienzle B, Ricci B, et al. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature. 1993;365:65–69. doi: 10.1038/365065a0. [DOI] [PubMed] [Google Scholar]
- Raabe M, Flynn LM, Zlot CH, Wong JS, Veniant MM, et al. Knockout of the abetalipoproteinemia gene in mice: Reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Natl Acad Sci U S A. 1998;95:8686–8691. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons GF, Wiggins D, Brown AM, Hebbachi AM. Synthesis and function of hepatic very-low-density lipoprotein. Biochem Soc Trans. 2004;32:59–64. doi: 10.1042/bst0320059. [DOI] [PubMed] [Google Scholar]
- Sehayek E, Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E- dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem. 1991;266:18259–18267. [PubMed] [Google Scholar]
- James RM, Scott AI, Dipak PR. Lipoprotein lipase: Structure, function, regulation, and role in disease. J Mol Med. 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- Perret B, Mabile L, Martinez L, Terce F, Barbaras R, et al. Hepatic lipase: Structure/function relationship, synthesis, and regulation. J Lipid Res. 2002;43:1163–1169. [PubMed] [Google Scholar]
- Ye J, Wang C, Sumpter R, Jr, Brown MS, Goldstein JL, et al. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci U S A. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Peng LF, Lin W, Choe WH, Sakamoto N, et al. A cell-based, high-throughput screen for small molecule regulators of hepatitis C virus replication. Gastroenterology. 2007;132:311–320. doi: 10.1053/j.gastro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Abe K, Yamada M, Dansako H, Naka K, et al. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–125. doi: 10.1002/hep.21232. [DOI] [PubMed] [Google Scholar]
- Wang C, Gale J, Keller BC, Huang H, Brown MS, et al. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Molecular Cell. 2005;18:425–434. doi: 10.1016/j.molcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Cardozo T, Pagano M. The SCF ubiquitin ligase: Insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- Kilsdonk EPC, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81:374–383. doi: 10.1128/JVI.01134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizaki H, Lee KJ, Sung VM, Ishiko H, Lai MMC. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology. 2004;324:450–461. doi: 10.1016/j.virol.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Evans MJ, Gosert R, Yuan Z, Blum HE, et al. Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. J Virol. 2004;78:7400–7409. doi: 10.1128/JVI.78.14.7400-7409.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Sun F, Owen DM, Li W, Chen Y, et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci U S A. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Date T, Miyamoto M, Zhao Z, Mizokami M, et al. Nonhepatic cell lines HeLa and 293 support efficient replication of the hepatitis C virus genotype 2a subgenomic replicon. J Virol. 2005;79:592–596. doi: 10.1128/JVI.79.1.592-596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Pellerin C, Lamarre D, Kukolj G. Hepatitis C virus subgenomic replicons in the human embryonic kidney 293 cell line. J Virol. 2004;78:491–501. doi: 10.1128/JVI.78.1.491-501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Guo JT, Seeger C. Replication of hepatitis C virus subgenomes in nonhepatic epithelial and mouse hepatoma cells. J Virol. 2003;77:9204–9210. doi: 10.1128/JVI.77.17.9204-9210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Zhang C, Chang KS, Jiang J, Ahn BC, et al. Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. J Virol. 2005;79:13963–13973. doi: 10.1128/JVI.79.22.13963-13973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AM, Huima-Byron T, Parker TS, Levine DM. Visualization of hepatitis C virions and putative defective interfering particles isolated from low-density lipoproteins. J Viral Hepat. 1996;3:11–17. doi: 10.1111/j.1365-2893.1996.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Andre P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SU, Bassendine MF, Burt AD, Martin C, Pumeechockchai W, et al. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol. 2006;80:2418–2428. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André P, Perlemuter G, Budkowska A, Bréchot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93–104. doi: 10.1055/s-2005-864785. [DOI] [PubMed] [Google Scholar]
- Pearse BMF. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc Natl Acad Sci U S A. 1982;79:451–455. doi: 10.1073/pnas.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger I, Schwab M, Stefanescu A, Hunziker W, Fuchs R. IgG transport across trophoblast-derived BeWo cells: A model system to study IgG transport in the placenta. Eur J Immunol. 1999;29:733–744. doi: 10.1002/(SICI)1521-4141(199903)29:03<733::AID-IMMU733>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hofer H, Bankl HC, Wrba F, Steindl-Munda P, Peck-Radosavljevic M, et al. Hepatocellular fat accumulation and low serum cholesterol in patients infected with HCV-3a. Am J Gastroenterol. 2002;97:2880–2885. doi: 10.1111/j.1572-0241.2002.07056.x. [DOI] [PubMed] [Google Scholar]
- Serfaty L, Andreani T, Giral P, Carbonell N, Chazouilleres O, et al. Hepatitis C virus induced hypobetalipoproteinemia: A possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428–434. doi: 10.1016/s0168-8278(00)00036-2. [DOI] [PubMed] [Google Scholar]
- Domitrovich AM, Felmlee DJ, Siddiqui A. Hepatitis C virus nonstructural proteins inhibit apolipoprotein B100 secretion. J Biol Chem. 2005;280:39802–39808. doi: 10.1074/jbc.M510391200. [DOI] [PubMed] [Google Scholar]
- Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta. 2000;1483:37–57. doi: 10.1016/s1388-1981(99)00182-1. [DOI] [PubMed] [Google Scholar]
- Deforges S, Evlashev A, Perret M, Sodoyer M, Pouzol S, et al. Expression of hepatitis C virus proteins in epithelial intestinal cells in vivo. J Gen Virol. 2004;85:2515–2523. doi: 10.1099/vir.0.80071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan FM, Chen AS, Hao F, Zhao FP, Gu CH, et al. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805–811. doi: 10.3748/wjg.v6.i6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz O, Delers F, Maynard M, Demignot S, Zoulim F, et al. Preferential association of hepatitis C virus with apolipoprotein B48-containing lipoproteins. J Gen Virol. 2006;87:2983–2991. doi: 10.1099/vir.0.82033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquerel L, Kuo CC, Dubuisson J, Levy S. CD81-dependent binding of hepatitis C virus E1E2 heterodimers. J Virol. 2003;77:10677–10683. doi: 10.1128/JVI.77.19.10677-10683.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- Koutsoudakis G, Herrmann E, Kallis S, Bartenschlager R, Pietschmann T. The level of CD81 cell surface expression is a key determinant for productive entry of hepatitis C Virus into host cells. J Virol. 2007;81:588–598. doi: 10.1128/JVI.01534-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- Cormier EG, Tsamis F, Kajumo F, Durso RJ, Gardner JP, et al. CD81 is an entry coreceptor for hepatitis C virus. Proc Natl Acad Sci U S A. 2004;101:7270–7274. doi: 10.1073/pnas.0402253101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, et al. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964–6972. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LJ, Brown SM. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunschmann S, Medh JD, Klinzmann D, Schmidt WN, Stapleton JT. characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J Virol. 2000;74:10055–10062. doi: 10.1128/jvi.74.21.10055-10062.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina S, Castet V, Fournier-Wirth C, Pichard-Garcia L, Avner R, et al. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J Hepatol. 2007;46:411–419. doi: 10.1016/j.jhep.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, et al. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- Grove J, Huby T, Stamataki Z, Vanwolleghem T, Meuleman P, et al. Scavenger receptor BI and BII expression levels modulate hepatitis C virus infectivity. J Virol. 2007;81:3162–3169. doi: 10.1128/JVI.02356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hahn T, Lindenbach BD, Boullier A, Quehenberger O, Paulson M, et al. Oxidized low-density lipoprotein inhibits hepatitis C virus cell entry in human hepatoma cells. Hepatology. 2006;43:932–942. doi: 10.1002/hep.21139. [DOI] [PubMed] [Google Scholar]
- Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisset C, Callens N, Blanchard E, Op De Beeck A, Dubuisson J, et al. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J Biol Chem. 2005;280:7793–7799. doi: 10.1074/jbc.M411600200. [DOI] [PubMed] [Google Scholar]
- Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, et al. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278:41624–41630. doi: 10.1074/jbc.M305289200. [DOI] [PubMed] [Google Scholar]
- Bartosch B, Verney G, Dreux M, Donot P, Morice Y, et al. An interplay between hypervariable region 1 of the hepatitis C Virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217–8229. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavillette D, Tarr AW, Voisset C, Donot P, Bartosch B, et al. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology. 2007;41:265–274. doi: 10.1002/hep.20542. [DOI] [PubMed] [Google Scholar]
- De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- Burnett JR. Drug evaluation: ISIS-301012, an antisense oligonucleotide for the treatment of hypercholesterolemia. Curr opin Mol Ther. 2006;8:461–467. [PubMed] [Google Scholar]
- Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- Chandler CE, Wilder DE, Pettini JL, Savoy YE, Petras SF, et al. CP-346086: An MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J Lipid Res. 2003;44:1887–1901. doi: 10.1194/jlr.M300094-JLR200. [DOI] [PubMed] [Google Scholar]