Abstract
Absolute pitch (AP) is the rare ability to identify the pitch of a tone without the aid of a reference tone. Understanding both the nature and genesis of AP can provide insights into neuroplasticity in the auditory system. We explored factors that may influence the accuracy of pitch perception in AP subjects both during the development of the trait and in later age. We used a Web-based survey and a pitch-labeling test to collect perceptual data from 2,213 individuals, 981 (44%) of whom proved to have extraordinary pitch-naming ability. The bimodal distribution in pitch-naming ability signifies AP as a distinct perceptual trait, with possible implications for its genetic basis. The wealth of these data has allowed us to uncover unsuspected note-naming irregularities suggestive of a “perceptual magnet” centered at the note “A.” In addition, we document a gradual decline in pitch-naming accuracy with age, characterized by a perceptual shift in the “sharp” direction. These findings speak both to the process of acquisition of AP and to its stability.
Keywords: perceptual magnet, pitch perception
The nature of absolute pitch (AP), also known as perfect pitch, lies outside the ken of most humans. It is an unusual perceptual gift, rigorously defined as the ability to name the pitch of a tone without the use of a reference tone. AP is distinguishable from relative pitch, a skill common in trained musicians, in which a pitch is rapidly derived by calculation of its interval from a reference pitch. AP can be loosely regarded as the musical equivalent of color labeling of visual frequencies, an ability common in humans.
Some questions regarding the quality, accuracy, and stability of AP have been considered (reviewed in ref. 1). Individuals with AP can readily and accurately classify the “chroma” (e.g., C, C#, D) of a pitch, often regardless of the musical or nonmusical source of the tone. AP appears to be more accurate on “white key” tones than “black key” tones, possibly reflecting the primacy of white keys in the key signatures used during early musical training. Some subjects with AP have anecdotally reported “musical paracusis,” a distortion in their pitch-naming brought on by influences such as drugs or age.
Similarly, the genesis of AP has been a subject of interest and speculation among musicians, psychologists, and neuroscientists (reviewed in refs. 1 and 2). AP provides an important model for understanding plasticity in the auditory system and serves as a paradigm for studying the interaction between nature and nurture, more generally, in the developing brain. Although early musical training has been correlated with acquisition of AP (3, 4), it is insufficient, in itself, to render AP. Recent technological and methodological advances have pointed instead to an inherent basis for AP, as first suggested by Bachem (5) and later bolstered by Profita and Bidder (6). For example, the exaggerated leftward asymmetry in the planum temporale of AP subjects compared with controls matched by musical training (7, 8) suggests a biological, rather than experiential, basis for this anatomic feature. Controlled heritability studies also provide strong support for genetic underpinnings to AP acquisition (9, 10). Together, these findings suggest a rare, inborn neurobiological template that can fertilize development of AP in the context of early musical exposure.
The present study attempts to shed more detailed insights into the nature of the perceptual ability itself and to reflect on the implications of the findings for understanding the neurodevelopmental aspects of pitch acquisition. We made use of the Web to enroll subjects efficiently into our study on absolute pitch. The Web proved to be an extremely effective recruitment tool, and the Web-based survey and pitch-naming test provided a vast digital archive of perceptual data. Here, we evaluate the perceptual data derived from this source to explore fundamental characteristics of absolute pitch.
Results and Discussion
Over a 3-year period (July 31, 2002 to July 31, 2005), subjects were recruited into the University of California Genetics of Absolute Pitch study through our website. Subjects were asked to identify both pure (synthesized sinusoidal) tones and piano tones, as described in Materials and Methods. There were 2,213 individuals who completed both the tone tests and the accompanying survey; of these, 981 (44%) exceeded the rigorous cutoff and were designated as “AP1” (see ref. 11 and Materials and Methods) on the basis of pure tone accuracy only.
Males and females were similarly represented in both the respondents (53% and 47%, respectively) and in those who tested AP1 (50% and 50%, respectively). Almost two-thirds (63%) of the subjects entering the study via the Web were between the ages of 10 and 29 years (overall median age of 20 years), almost certainly reflecting facility of this age group with the Web. Self-reporting of AP ability proved fairly reliable: 77% of those who claimed to have AP actually scored AP1; conversely, of the 981 subjects who tested AP1, 89% self-reported AP in the survey, and, of those who failed to test AP1, only 23% self-reported AP. Neither AP1 nor non-AP1 subjects were musically naïve, with 89% and 67%, respectively, claiming 6 or more years of musical training.
Dichotomous Distribution of Pitch-Naming Scores.
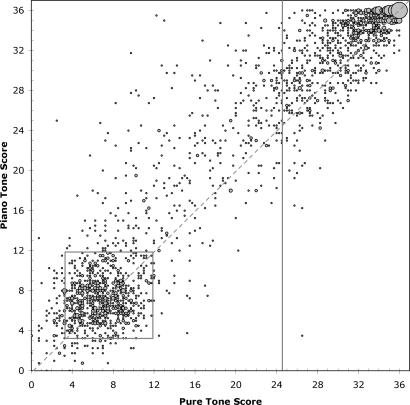
Fig. 1 shows the distribution of scores from all participants in this study. The relationship between pure and piano tone accuracy is roughly linear, with most scores falling close to the diagonal. Subjects tended to perform slightly better on piano tones than pure tones, suggesting that overtone and timbre cues augment their correct assignment: the mean difference between piano and pure tone scores is 1.55, with a 95% confidence interval of 1.39 to 1.71 (P < 0.0001).
Fig. 1.
Scatter plot of piano tone scores as a function of pure tone scores. Data from 2,213 subjects who completed the survey and acoustical test online are presented. The vertical gray line indicates the cutoff value of 24.5 for our operational definition of AP1, as defined in ref. 11. The dashed diagonal line indicates a theoretical 1:1 correlation between pure and piano tone responses. The gray box indicates the range of scores expected by chance distribution, with mean expected score by chance of 7.125 and with 95% of expected values lying between scores of 3.25 and 11.75. The area of the circle for each data point is proportional to the number of individuals scoring at that point.
Although pure and piano tone accuracy are roughly concordant within an individual, the distribution in subjects' scores was not unimodal across the range of possible scores. Rather, the scores clustered into two groups: those that represent highly accurate pitch perception (AP1) and those that are consistent with the expected distribution for random assignment (Fig. 1, gray box). Note that the probability of testing AP1 by chance alone is 1.53 × 10−12 (see Materials and Methods for calculation). This striking, bimodal distribution resolves the question of whether AP ability lies in the tail of a continuous perceptual spectrum or, rather, defines a distinct perceptual trait. Our data, which demonstrate that pitch-naming ability is a dichotomous trait, clearly support the latter.
This finding contrasts with the observations of many complex traits, such as stature and blood pressure, whose normal distributions likely reflect the additive effect of many genes. Thus, the data from Fig. 1 suggest the possibility that AP ability could be governed by the influence of only one or a few genes. In this model, AP could result from inheritance of predisposing alleles with significant impact, modulated by early exposure to music.
Visualization of Perceptual Warps in Absolute Pitch Subjects.
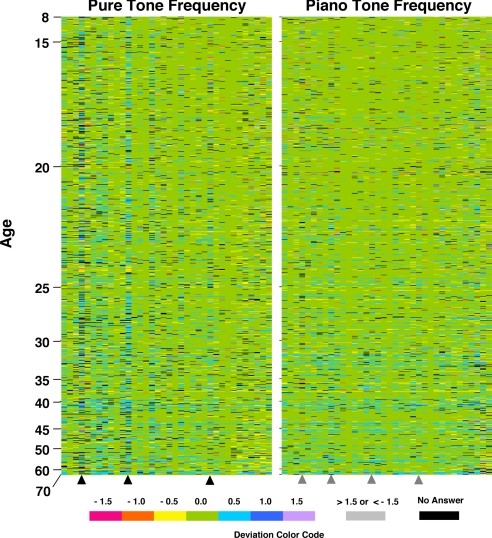
Fig. 2 shows the distribution of pitch errors made by the subjects who tested AP1, as a function of age and pitch. Deviations in pitch assignments are represented by colors, as shown by the key, so that patterns in deviations may be easily discerned by visual inspection. Subject ages ranged from 8 to 70 years, and the presented tones span six octaves. The field of green represents no error in pitch naming, whereas blue and purple hues convey errors in the “sharp” direction, and yellow and red hues convey errors in the flat direction. Data are presented for both pure and piano tones. Two conclusions can be readily drawn from these data and are examined in more detail below.
Fig. 2.
Visual representation of pure and piano tone deviations as a function of age and of pitch frequency. Flat (negative) and sharp (positive) deviations are color-coded, as indicated in the key, with a value of 0.5 per semitone deviation from the correct response (0 deviation, coded green). Errors of greater than three semitones in either direction are coded gray, and failure to assign a tone within 3 seconds is coded black. Data are summarized for 981 subjects, with an age range of 8–70, who tested AP1. Frequencies are arranged in ascending order from left to right. Scored pure tone frequencies range from D#2 to B7 (77.78–3,951.10 Hz), and piano tone frequencies range from C#2 to A7 (69.30–3,520.00 Hz). Black and gray arrowheads indicate G#'s in pure and piano tone tests, respectively.
Displacement of the Frequency Place-Map with Age.
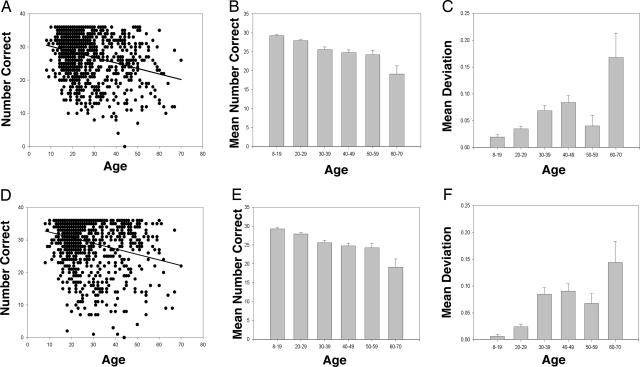
First, pitch errors increase with age, and they tend to be sharp. This trend can be visualized as a “pile-up” of blue color at the bottom of the color-coded deviation data in Fig. 2. In a mathematical presentation of the same data, Fig. 3(A and D) illustrates the declining trend in the average number of correctly identified notes (i.e., with a deviation of 0) with age. This trend is significant: Spearman correlation coefficients show for pure tones, r = −0.251 (95% confidence interval of −0.309 to −0.192, P < 0.0001), and for piano tones, r = −0.244 (95% confidence interval of −0.302 to −0.184, P < 0.0001). Indeed, no subject older than 51 identified all 36 tones in both tests correctly, and only three answered all piano tones correctly. This displacement is also substantiated by pitch perception data (data not shown) obtained through compact disk test of 48 AP1 subjects (median age of 48) who had difficulty accessing the Web test.
Fig. 3.
Pitch perception as a function of age for pure (A–C) and piano (D–F) tones. (A and D) The number of correct pure (A) and piano (D) tone responses plotted as a function of age for the 981 AP1 subjects. Some data are obscured by superimposition of data points. The lines represent the regression, in which Pearson correlation coefficients are −0.251 (with a 95% confidence interval of −0.309 to −0.192, P < 0.0001) and −0.244 (95% confidence interval of −0.302 to −0.184, P < 0.0001) for pure and piano tones, respectively. (B and E) The decline in pitch-naming ability can be visualized. The mean correct responses by age clusters are summarized as follows: 8–19 (n = 319), 20–29 (n = 374), 30–39 (n = 130), 40–49 (n = 100), 50–59 (n = 45), and 60–70 (n = 13). (C and F) The data for the algebraic means of the deviations for all responses within each age group (as above), where a correct response is assigned a value of 0 (no deviation), semitone sharp and flat errors are assigned values of +0.5 and −0.5, respectively, whole tone sharp and flat errors are assigned values of +1.0 and −1.0, respectively, etc. The tritone, which was rarely assigned, was given the value of +3. Unnamed pitches were discarded, and, in calculating the mean, the denominator was decreased from 36 by the number of discarded notes. Error bars indicate SEM.
This correlation also supports the rationale for giving partial (3/4 point) credit for an answer deviating by one semitone. This adjustment allowed for the capture of subjects, particularly of advanced age, who may have exceptional pitch perception but who may be systematically sharp or flat by a semitone. For example, one subject of age 44 failed to correctly identify a single pure or piano tone but instead responded with a semitone sharp for every note, thus enabling him to be classified as AP1; he had recognized his tendency for sharp perception at age 22. One can see that indeed the partial credit approach was of value, because the average number of correctly identified tones drops by ≈33% from the youngest to the oldest age groups (Fig. 3 B and E). Fig. 3 C and F summarizes the enhanced tendency of subjects to misidentify tones in the sharp direction as they age, as noted by the positive value deviation.
These findings dramatically substantiate the anecdotal reports of some AP possessors who bemoan a diminution in their pitch accuracy in later life (reviewed in ref. 1). For example, in describing his own disconcerting shift in pitch-naming ability at age 52, Vernon (12) suggested that a change in the elasticity of the basilar membrane of the cochlea might underlie this shift in perception. Indeed, we reason that an increase (not a decrease, as suggested by Vernon) in the elasticity of the basilar membrane would be predicted to cause a displacement in the cochlear frequency map in the sharp direction. In this model, hair cells that formerly resonated for a given tone (e.g., E) and relayed that stimulus to the auditory cortex now respond at a lower frequency (e.g., D#). Because the hair cells that are triggered by this lower frequency remain hard-wired to relay a signal to a higher frequency recognition site in the auditory cortex, one perceives the tone at a higher frequency.
Any age-dependent physiological change that alters the mechanical properties of the cochlea might underlie this phenomenon. For example, the age-related decline in the number of mesothelial cells along the length of the basilar membrane (13) could provide one such mechanism. These cells are the likely source of fibronectin and other extracellular matrix proteins, which largely influence the stiffness and mass of the basilar membrane and thus define where it is maximally excited for each frequency (14). Diminution in mesothelial cell density might reasonably be expected to cause displacement of the frequency map of the cochlea with age by changing its mechanical properties.
Thus, by analysis of AP1 individuals, we can document a gradual, age-dependent distortion in frequency perception in the auditory pathway. We hypothesize that such a gradual perceptual shift is common to most people as they age, yet they are unaware of it unless they have AP. Although we have not yet tested this hypothesis by audiometry, we reason that the perceptual shift is distinguishable from sensory presbycusis, a high-frequency hearing loss associated with advanced age and primarily due to loss of hair cells in the basal cochlea (15).
The age-dependent shift in the cochlear frequency map may be conceptually similar to age-dependent changes in the visual system, such as the onset of presbyopia (far-sightedness) that ensues in the fifth decade of life. Perhaps a more apt analogy is the shift in color perception brought on by lens opacity with age. Although older individuals are unaware of the gradual distortion during cataract development, they experience a shift in color perception upon lens replacement (16). It is interesting to consider that AP subjects provide a unique window into these physiological changes.
Perceptual Magnet Effect.
The second set of conclusions to be drawn from the color-coded deviation data presented in Fig. 2 concerns pitch-naming accuracy among AP1 individuals across all age groups as a function of either pitch “height” or pitch “class.” Pitch height, often referred to as “octave,” describes whether a pitch is high or low on the spectrum of pitch frequencies, whereas pitch class, also referred to as chroma, describes the note assignment of each of the 12 notes of the Western scale (A, A#, B, etc.), regardless of octave.
We did not observe a consistent or significant effect of pitch height (spanning six octaves) on pitch-naming ability (data analysis not shown). In contrast, however, we discovered that pitch-naming errors are not uniform among pitch classes. The most prominent distortion manifests as three columns of blue (indicated by black arrowheads in Fig. 2) corresponding to semitone sharp error responses to all three G#s given in the pure tone test. Interestingly, deviations in G# assignments are not readily apparent for piano tones (indicated by gray arrowheads in Fig. 2).
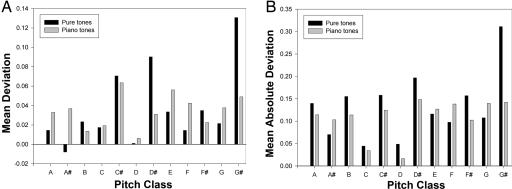
Fig. 4 summarizes the data and substantiates these impressions. The mean deviation in named pitch for all AP1 subjects is shown for each pitch class (Fig. 4A). As described above, deviations tend to be in the sharp (positive) direction, with a tendency to err more frequently on sharps (black keys on a standard piano) than “naturals” (white keys), a phenomenon noted previously by Miyazaki (17) for synthesized piano tones and reviewed by Ward (1). Here, for piano tones, the pair-wise difference between mean percentage correct for white and black notes is 3.46 (95% confidence interval 2.33–4.59, P < 0.0001). The tendency is more exaggerated for pure tones, the pairwise difference being 12.94 (95% confidence interval 11.08–13.91, P < 0.0001).
Fig. 4.
Pitch deviation as a function of pitch chroma. The mean deviations (A) and mean absolute deviations (B) in pitch identification (as calculated in Fig. 3) are plotted for each pitch. The distribution of pitches is given in Materials and Methods.
However, the amplitude of the deviations varies widely by pitch class and by tone source (pure vs. piano). Two extremes illustrate this phenomenon: given as a pure tone, G# is as perceived sharp far more than any other tone, whereas errors in D occur infrequently, regardless of tone source (as confirmed by mean absolute deviations in Fig. 4B). Interestingly, pure A# is most often perceived as flat, not in keeping with the other pitches, a finding that has further implication (see below).
A statistical analysis shows that G# is uniquely error-prone. Pair-wise comparisons of deviations in responses to all pure tones reveal significant differences in all comparisons involving G#'s (Bonferroni adjusted P values all <0.003). No other tone shares this property.
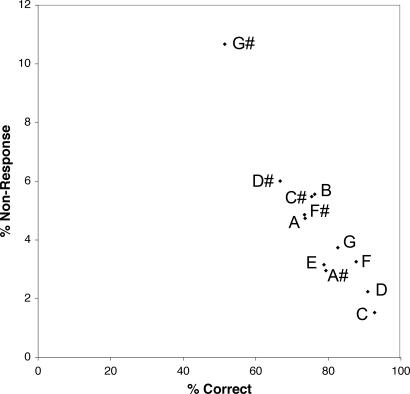
The frequent misidentification of pure G# bears further scrutiny. As shown in Fig. 5, AP1 subjects correctly identified pure G# only 52% of the time, and 11% of the G# cues failed to be named at all; these responses are in marked contrast with those for other pitches. Moreover, 26% of the presented G#s are misidentified as A (see below).
Fig. 5.
Relationship between nonresponses and correctly identified responses for each pitch chroma. Plotted are percentages of pitch cues unanswered as a function of percentages of pitch cues correctly identified for each pitch class. Data are compiled from responses from 981 AP1 subjects.
What might account for this perceptual bias? At least part of the explanation for the G# error could lie in the use of A as the universal tuning frequency. Orchestras tune to an A over a fairly wide frequency interval, from A415 in early music to A446 in the Berlin Philharmonic. Musicians and concert-goers are thus exposed to a wide range of tuning A pitches, and those with AP may have learned to accommodate to this broad spectrum, capturing both the presented G# and, to a lesser extent, A# (415 and 466 Hz, respectively) within the A category. The general tendency to misidentify pitches in the sharp direction exacerbates the binning of G# into the A category and makes the A# misperception in the flat direction even more compelling. Significantly, this phenomenon is eliminated in the context of overtone and timbre cues in piano tones; because most pianos are tuned to A440, there is no experiential need to accommodate the piano tone A to other frequencies.
This widening of the “bin” for frequencies perceived as A can be likened to a perceptual magnet effect previously described for speech (18, 19). The perceptual magnet is a concept used to explain the relative inability to discern variations around a prototypic vowel sound, as compared with variations around a nonprototypic vowel sound. The perceptual magnet effect is a consequence of exposure to a specific language during infancy, when babies learn to bin ranges of vowel sounds into categories delineated by their native language (20). Although the experimental paradigm used in the current study is not equivalent to that used in the speech perception experiments, the effect is the same, namely, that the perception of a tone (G#) is distorted by its neighboring tone (A), which has prominent exposure in Western music. The properties of perception centered on A, as uncovered in our study, as well as the role of A in musical tuning combine to make this a compelling hypothesis.
Future studies from our group and others should help to refine the concept of the perceptual magnet in music. We are particularly curious to resurvey our study population and to engage non-Western populations to determine whether a correlation exists between the perceptual magnet effect and the musical exposure (such as the performance instrument, training, or musical sphere) of the AP subject.
Coda.
Although we developed the Web-based study as a recruitment tool for our genetic study, we now appreciate the value of this approach for collecting and analyzing auditory data. Although subjects in this study were not tested under conventional laboratory settings, the nature of the pitch errors uncovered herein vigorously upends the possibility that subjects are prone to employ pitch-naming aids in the Web-testing paradigm. Moreover, our findings recapitulate and extend the observations derived either anecdotally or from studies typically involving very few subjects. With careful application, such a Web-based strategy should prove effective for further studies on pitch perception.
The number of AP subjects who have been drawn into this study via the Web far exceeds that for any previous AP study. Even without remuneration, the subjects have been engaged in this process, curious about the origins and properties of their own AP abilities, and eager to share by e-mail life histories, musical insights, and points of discussion for future studies.
Materials and Methods
Subject Recruitment.
We recruited subjects into our study via the Web. Subjects became aware of our Web site through news reports on our project, advertisements placed in music teachers' and professional musicians' journals, fliers sent to music schools, word-of-mouth, and Web surfing.
Participants filled in a short questionnaire on their pitch ability, musical training, and relatives who may have AP. The questionnaire can be viewed online at the University of California Absolute Pitch Study web-site.
Subjects were given a pitch identification test online. This test, which was developed and described in detail in Baharloo et al. (11), consists of 40 randomly selected pure tones (i.e., computer-generated sine waves without overtones) and 40 randomly selected piano tones (digitized from a tuned Steinway piano) taken from the spectrum of frequencies on a piano keyboard.
We ruled out errors inherent in our study by verifying (with programs Matlab and Buzz-O-Sonic 3.0) that the pure and piano tones produced both over the Web (using both Netscape and Internet Explorer browsers) and by compact disk were precisely as defined by a tempered scale tuned to A440.
The pitch identification test requires the subject to click on a screen keyboard in response to a series of presented tones. A brief practice test was given first to acquaint subjects with the keyboard response on the computer screen and to adjust the volume of their computers. Tones were given for a duration of 1 second with a 4-second interlude between tone onsets and were delivered in a series of 10 tones, giving the subject an opportunity to pause between sets. Because four of the pure tones lie above the piano keyboard and four of the piano tones lie in the lowest keyboard octave, and thus proved difficult to hear, these were excluded from the scoring. The chromatic distribution of the 72 scored tones (n pure, n piano) was as follows: C (3, 3), C# (4, 4), D (3, 1), D# (4, 4), E (2, 2), F (3, 3), F# (4, 4), G (2, 3), G# (3, 4), A (4, 3), A# (1, 4), B (3, 1).
The entire procedure (questionnaire plus pitch-identification test) required ≈20 minutes. Subjects were advised that, by completing the survey and providing contact information, they were giving to participate in the study. This protocol was approved by the Committee on Human Research at the University of California, San Francisco.
Scoring Algorithm.
The pure tone test and piano tone test were scored separately. Subjects were given 1 point for each correct answer (maximum score for each test is 36) and 3/4 point for each error of a semitone. Those who scored above a threshold of 24.5 in pure tones were designated AP1. This stringent criterion allowed for inclusion of subjects with only exquisitely accurate pitch perception.
In the paradigm of Baharloo et al. (11), semitone errors in subjects age 45 years and older were given full credit rather than the 3/4 credit awarded younger subjects. In the current study, we did not apply this handicap in scoring tests so as not to oversample pitch deviations in older subjects and thus bias the outcome. Removal of the handicap eliminated 23 people who would otherwise have met criteria for the genetic study.
Statistical Analyses.
Statistical analyses are as follows: comparisons of pure and piano tone scores were done with paired t tests. Pearson and Spearman correlations were used for associations of age and number of correct responses. For comparisons of accuracy as a function of pitch class, a person's accuracy for a particular pitch class was defined as the average number of whole tone differences between the responses for that pitch and the correct pitch. For example, if G# is presented three times and the responses are A, G#, A#, then the mean deviation is (0.5 + 0 + 1)/3 = 0.5.
Comparisons of deviations between pitch classes used a mixed-effects regression model with a random-subject effect and fixed-pitch class effect with 12 levels. Pair-wise comparisons of mean deviations to G# with mean deviations to other pitch classes used a Bonferroni correction (P values multiplied by 11).
The pure tone or piano tone score was computed as score = X + 0.75Y, where X is the number of correctly identified tones and Y is the number of semitone errors. If responses are random, the distribution of X is binomial with n = 36 and p = 1/12. The distribution of Y (conditional on X) is binomial with n = 36 − X and p = 2/12 because there are two ways to make a semitone error. For example, the probability that X = 5 and Y = 3 is C536 (1/12)5(11/12)31 × C331 (2/12)3(10/12)28, where Cmn is the binomial coefficient (n!/(m!(n − m)!). This probability can be computed for each possible combination of X and Y (X = 0, …, 36, Y = 0, … N–X). Then, the probabilities for X,Y combinations that generate the same score can be summed to get the probability distribution over the possible scores. This calculation yields a total of 1.53 × 10−12 for the scores larger than 24.5 (the cutoff frequency for AP1). The mean expected score by chance is 7.125 with 95% of expected values lying between scores of 3.25 and 11.75.
Acknowledgments
We thank Beth Theusch, Michael Brainard and members of his laboratory, Allison Doupe, Neil Risch, Patricia Kuhl, Diana Deutsch, Ken'ichi Miyazaki, Siamak Baharloo, Peter Gregersen, Victoria Carlton, and the University of California, San Francisco, Genetic Epidemiology Group for many helpful insights into this study; Aaron Calhoun for web site development and maintenance; Julie Bernstein and the University of California, San Francisco, Public Relations Office for their interest and support in this project; and the many subjects who took the time to enter our study and correspond with us. These studies were supported by the Howard Hughes Medical Institute and a Guggenheim Memorial Fellowship (to J.G.).
Abbreviation
- AP
absolute pitch.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 14549.
References
- 1.Ward WD. Deutsch D. The Psychology of Music. 2nd Ed. San Diego: Academic; 1999. pp. 265–298. [Google Scholar]
- 2.Zatorre RJ. Nat Neurosci. 2003;6:692–695. doi: 10.1038/nn1085. [DOI] [PubMed] [Google Scholar]
- 3.Sergeant D. J Res Music Education. 1969;17:135–143. [Google Scholar]
- 4.Takeuchi AH, Hulse SH. Psychol Bull. 1993;113:345–361. doi: 10.1037/0033-2909.113.2.345. [DOI] [PubMed] [Google Scholar]
- 5.Bachem A. J Acous Soc Am. 1955;27:1180–1185. [Google Scholar]
- 6.Profita F, Bidder TG. Am J Hum Genet. 1988;29:763–771. doi: 10.1002/ajmg.1320290405. [DOI] [PubMed] [Google Scholar]
- 7.Schlaug G, Jancke L, Huang Y, Steinmetz H. Science. 1995;267:699–701. doi: 10.1126/science.7839149. [DOI] [PubMed] [Google Scholar]
- 8.Keenan JP, Thangaraj V, Halpern AR, Schlaug G. NeuroImage. 2001;14:1402–1408. doi: 10.1006/nimg.2001.0925. [DOI] [PubMed] [Google Scholar]
- 9.Baharloo S, Service SK, Risch N, Gitschier J, Freimer NB. Am J Hum Genet. 2000;67:755–758. doi: 10.1086/303057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregersen PK, Kowalsky E, Kohn N, Marvin EW. Am J Hum Genet. 1999;65:911–913. doi: 10.1086/302541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baharloo S, Johnston PA, Service SK, Gitschier J, Freimer NB. Am J Hum Genet. 1998;62:224–231. doi: 10.1086/301704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernon PE. Br J Psychol. 1977;68:485–489. doi: 10.1111/j.2044-8295.1977.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt KA, Liberman MC, Nadol JB. Ann Otol Rhinol Laryngol. 2001;110:1147–1153. doi: 10.1177/000348940111001212. [DOI] [PubMed] [Google Scholar]
- 14.Keithley EM, Ryan AF, Woolf NK. J Comp Neurol. 1993;327:612–617. doi: 10.1002/cne.903270411. [DOI] [PubMed] [Google Scholar]
- 15.Maurer JF, Rupp RR, editors. Hearing and Aging: Tactics for Intervention. New York: Grune & Stratton; 1979. pp. 47–50. [Google Scholar]
- 16.Delahunt PB, Webster MA, Lei M, Werner JS. Visual Neurosci. 2004;21:301–307. doi: 10.1017/S0952523804213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazaki K. Percept Psychophys. 1988;44:501–512. doi: 10.3758/bf03207484. [DOI] [PubMed] [Google Scholar]
- 18.Grieser D, Kuhl PK. Dev Psychol. 1989;25:577–588. [Google Scholar]
- 19.Kuhl PK. Percept Psychophys. 1991;50:93–107. doi: 10.3758/bf03212211. [DOI] [PubMed] [Google Scholar]
- 20.Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindblom B. Science. 1992;255:606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]